
Novel Access Towards Chiral α-trifluoromethyl Alcohols
Fluorine-containing organic compounds are of high interest to the chemical community, as fluorinated molecules exhibit significantly different chemical and physical properties, comparing to non-fluoriated analogs.1 One of the important families of fluorinated compounds are α-trifluoromethyl alcohols. These compounds, in their enantiopure form, have found wide and important applications, especially in bio-medical and pharmaceutical fields.2,3 Current methods for the preparation of these alcohols are based on enantioselective reduction of the corresponding trifluoromethyl ketones using expensive chiral boranes or chiral iridium catalysts.
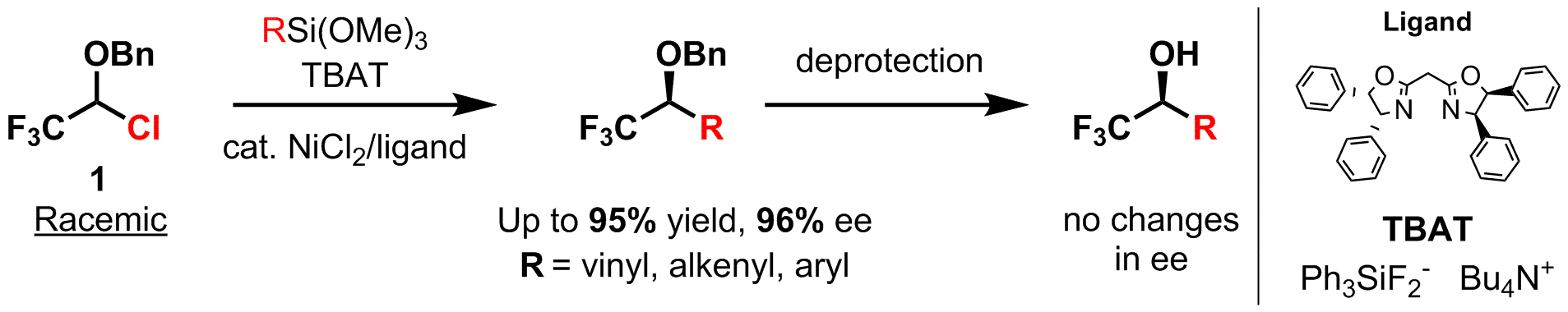
We have developed an approach to access these compounds via completely different disconnection utilizing an easily prepared substrate 1 as a starting material. Performing the asymmetric stereoconvergent cross-coupling reaction with this building block, containing protected alcohol group at the reaction center, we can prepare (after deprotection) a target CF3-substituted alcohol in a facile and efficient manner.
Namely, applying nickel-catalyzed Hiyama cross-coupling reaction to the substrate 1, various protected allyl- or benzyl(α-trifluoromethyl) alcohols could be obtained in excellent yields and enantioselectivity (ee), while during further deprotection of the alcohol ee remains unaffected.
References
- Alonso, C.; Martinez de Marigorta, E.; Rubiales, G.; Palacios, F.; Chem. Rev. 2015, 115, 1847-1935
- Garcia-Martinez, C.; Taguchi, Y.; Oishi, A.; Hayamizu, K.; Magn. Reson. Chem., 1998, 36, 429-435
- Goldberg, R.D.; De Lombaert, S,; Aiello, R.; Bourassa, P.; Barucci, N,; Zhang, Q.; Paralkar, V.; Valentine, J.; Zavadoski W.; Bioorg. Med. Chem. Lett., 2016, 26, 1124-1129.

Powered by Eventact EMS