
Sunscreen Assisted Photochemical Divergence
2Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva
Many photochemically induced processes are essential for the existence of life; but other biochemical reactions triggered by UV light may lead to DNA damage and degradation. To protect us from this, chemists have developed sunscreens in the form of external creams which deflect or absorb these harmful radiations. Moreover, some organisms are able to produce special molecules that absorb light to prevent harmful events, and there is even speculation that the highly efficient UV absorption by nucleic bases was one of the selection processes that determined how life on earth evolved. Inspired on this, we thought it would be an appealing goal to achieve control over photochemical synthetic pathways, depending on whether molecular sunscreens are present or not.
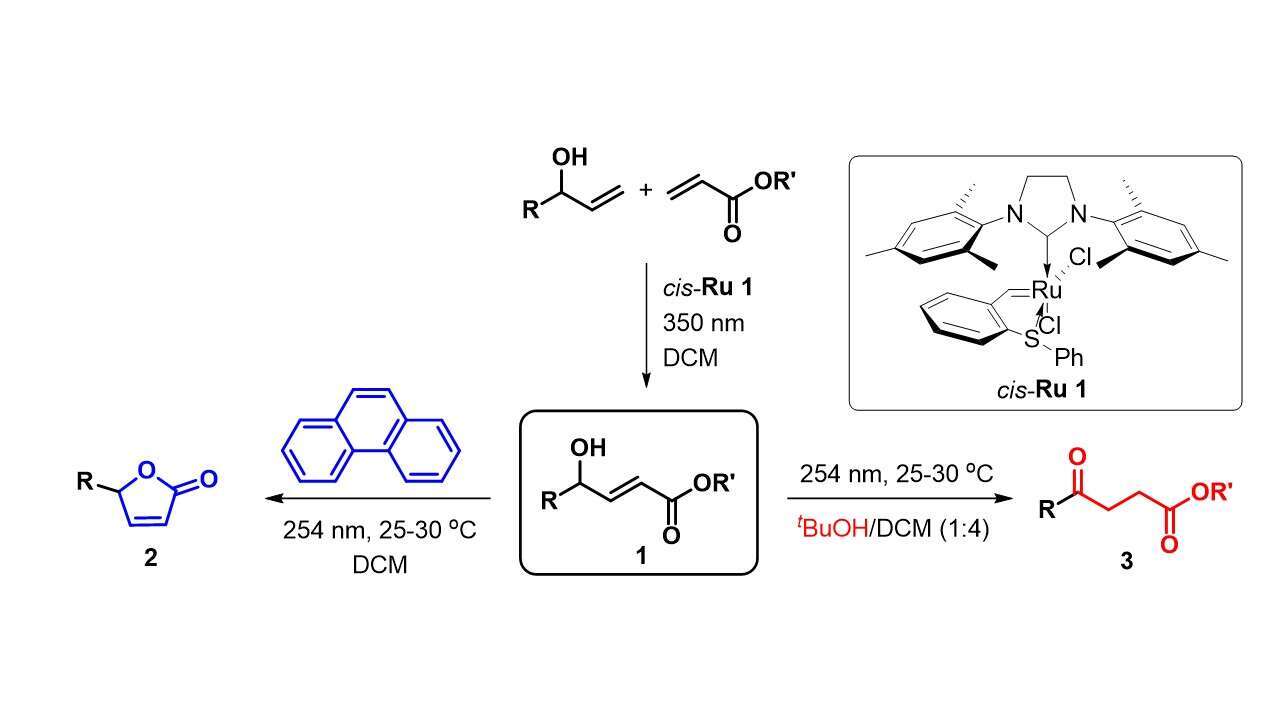
In this study, we explore the property of light-absorbing organic molecules that can screen UV-irradiation to achieve selective organic transformations. Thus, by starting out with simple allyl alcohols and acrylates, sequential photoinduced cross-metathesis (UV-A), followed by UV-C irradiation in the presence of latent catalyst cis-Ru 1 led to butenolides (2) or levulinates (3). Several examples of the photo-chemo-selective pathway are demonstrated, including the synthesis of two natural products and important precursors towards different natural products simply by UV-irradiation either in the presence or absence of a molecular sunscreen.

Powered by Eventact EMS