
The Rat 50/10 Oxygen-induced Retinopathy as a Potential Model of Bronchopulmonary Dysplasia
Background: Bronchopulmonary dysplasia (BPD) is a chronic lung disease that affects preterm babies, characterized by abnormally greater amount of myofibroblast and alveolar walls overwhelmed by collagen. Rodent models of BPD are dominated by hyperoxia insults, while these models are in urgent need of modification due to that pure hyperoxia is not consistent with the clinical status in most of the NICUs nowadays. The rat 50/10 oxygen-induced retinopathy (OIR) model is used in the study of retinopathy of prematurity (ROP) and becoming widely accepted these years resulting from its consistency with clinic.
Objective: In pursuit of a better model of BPD, we conducted OIR and analyzed the pathological changes of lungs.
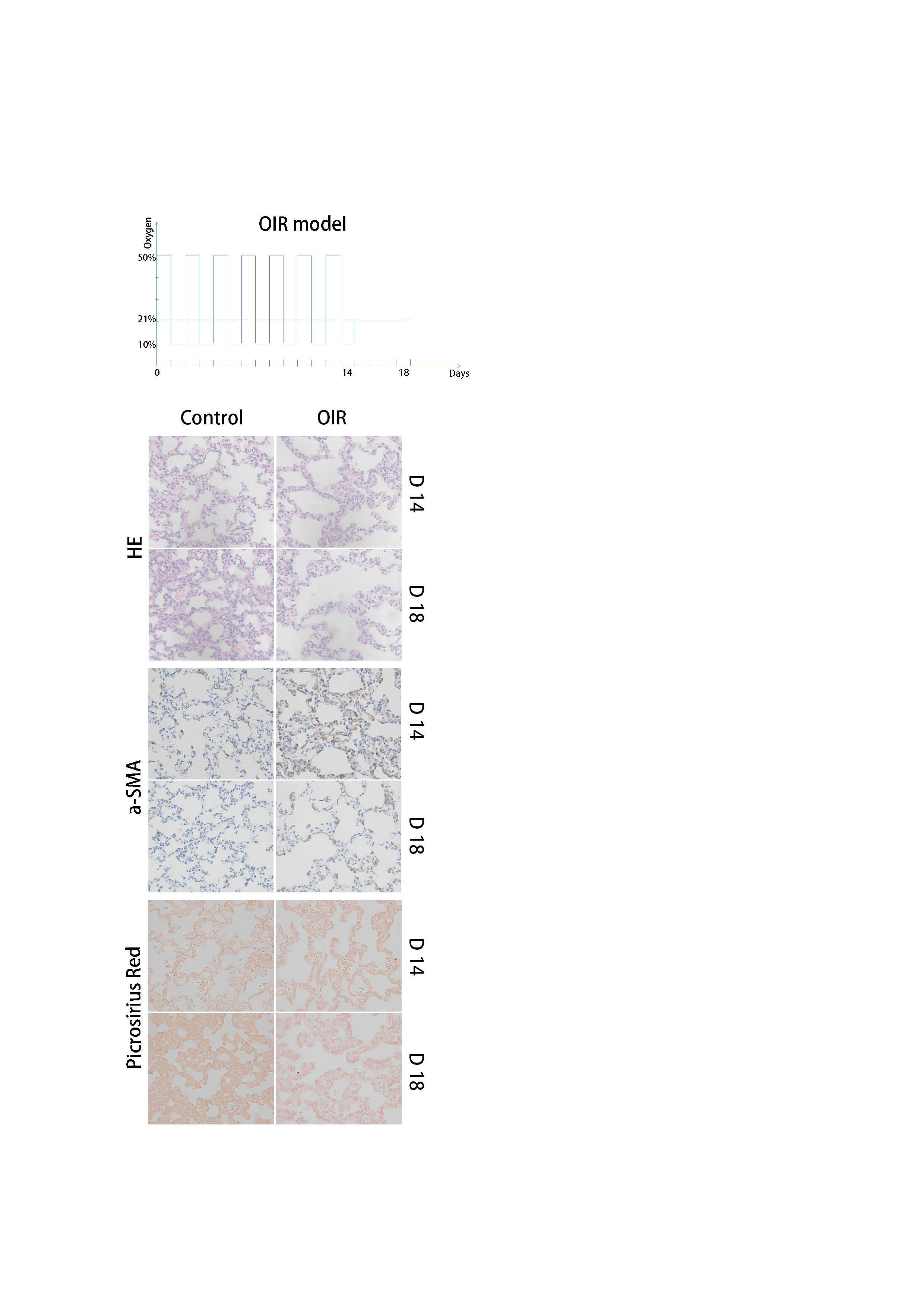
Methods: The rat OIR model was conducted by putting the newborn rat into cycled 50% and 10% oxygen (from the day of birth, each concentration for 24 hours), lasting for 14 days and then exposed to normoxia for another 4 days. Lungs were harvested at both day 14 (D14) and D18 for HE, α-SMA and Picrosirius Red staining.
Results: At D14, lungs in the OIR group presented with fewer and enlarged alveolar. At D18, the alveolar enlarged further and the septa thickened. In α-SMA, it was higher in OIR groups, indicating more myofibroblast localization. At D18, when myofibroblast was almost absent from the alveolar walls in control group, the OIR lungs had persistence expression of α-SMA. In Picrosirius red, the amount of collagen in OIR group was higher than that of control group at both time points and the trend was more obvious at D18.
Conclusion: The prolonged and stronger existence of myofibroblast produced and intensified collagen deposition in the OIR group, which was similar to other BPD animal models. Combining the positive manifestations of HE staining, the rat OIR has potential in serving as a new model of BPD.

Powered by Eventact EMS