
Pathogen Specific Changes in Core Temperature and Neuro-Inflammation in The Neonatal Rat
2Department of Paediatric Neurology, University Children’s Hospital
3Department of General Paediatrics, Neonatology and Paediatric Cardiology, University Children's Hospital, Heinrich-Heine University
4School of Clinical Sciences, University of Bristol
Background: Perinatal infections increase vulnerability of the neonatal brain to hypoxic-ischemic (HI) injury. We recently demonstrated experimentally how inflammatory pre-sensitisation through TLR-4 (LPS) abolished the effect of therapeutic hypothermia (TH) after HI. Remarkably, when inflammation is induced through TLR-2 (PAM3CSK4), TH provides 80% neuroprotection1,2. A sepsis-like condition is induced in both situations, but how they differ with respect to neuro-inflammatory responses in the newborn is unknown.
Objective: To examine physiological and neuro-immunological changes induced in the neonatal rat by triggering inflammation systemically using LPS or PAM3CSK4 (PAM), compared to saline.
Methods: After a systemic injection of 0.9% NaCl (Veh), LPS, (0.1mg/kg) or PAM (1 mg/kg) in P7 rats, core temperature was measured 2-hourly for 24h. Brain tissue was analyzed for pro-inflammatory cytokines (IL-6, IL-1β, TNF-α, and IL-10) (PCR) and apoptosis (cCas3) and microglial activation (Iba1) (WB).
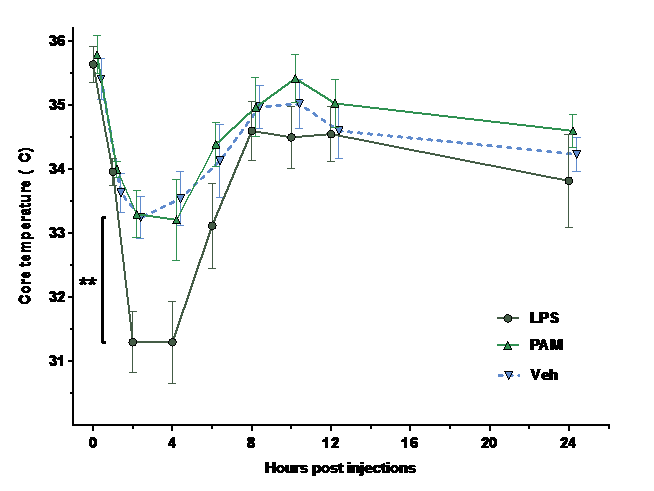
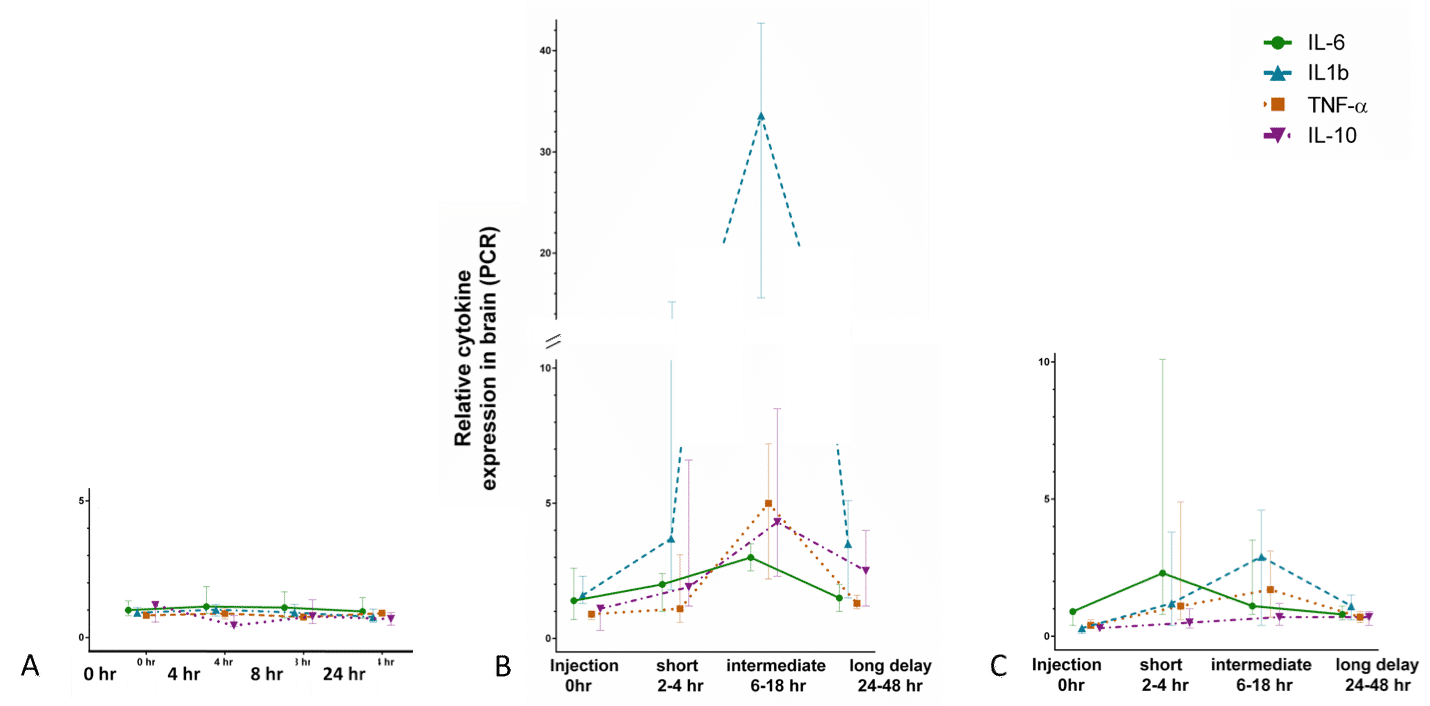
Results: LPS induced a significant drop in core temperature after injection compared to Veh (p=0.01) and PAM (p=0.01) (Fig 1). Temporal expression of IL-6, IL-1β, TNFα and IL-10 differed significantly after injection of LPS and PAM, with expression peaks after different incubation lengths (Fig 2, A: PAM, B: Veh, C: LPS). cCas3 was significantly increased 24-48 hours after LPS injection compared to PAM and Veh (p=0.0002) which were similar. Iba1 was increased by systemic LPS and PAM compared to Veh (p=0.07).
Conclusion: Experimental induction of a sepsis-like condition through TLR-2 and TLR-4 induced neuro-inflammation. The gram-positive pathway (PAM) creates a neuro-inflammatory state very different from that of the gram-negative pathway (LPS). The difference in core temperature preservation indicates a role of metabolic demands during infection that might be pathogen specific. These results highlight the need for more research on inflammatory cross-talk between blood and brain, and the importance of pre-clinical models being carefully tailored to their clinical scenario.
|
1. Osredkar, D. et al. Resuscitation 85, 567–572 (2014). |

Powered by Eventact EMS