
Bilistick® System as a Reliable, Cost-effective Tool for Predicting the Need of Treatment for Hyperbilirubinemia in Low Middle Income Countries
2Neonatology Division, Child Health Department, Cipto Mangunkusumo Hospital - University of Indonesia
3Research Department, Firetree Asia Foundation
4Department of Paediatrics, Cairo University Children Hospital
5Department of Paediatrics, Faculty of Medical Sciences, University of Jos, Jos university Teaching Hospital
6Department of Paediatrics, Lagos University Teaching Hospital
Background: In many Low Middle Income Countries, the access to laboratory support to estimate promptly bilirubin levels in jaundiced newborns is very difficult. This results in a delayed assessment of potentially dangerous jaundice increasing the risk of kernicterus. Implementation of inexpensive and simple devices to evaluate hyperbilirubinemia and treatment needs are essential to prevent neurological damages. We performed a multicenter study in 21 hospitals serving ethnically different populations distributed in Indonesia (7), Vietnam (9), Nigeria (4) and Egypt (1) to evaluate the assessment of the low cost Point-of-Care bilirubin assay Bilistick® System (BS) in determining hyperbilirubinemia and treatment needs according to NICE guidelines.
Objective: To determine the effectiveness of BS to assess neonatal hyperbilirubinemia and indicate the appropriate treatment according to NICE guidelines.
Methods: Blood samples were obtained in 1016 healthy ≥36 weeks of gestation newborns, presenting visual symptoms of neonatal jaundice. Bilirubin level was assessed by laboratory and BS methods; age of the patient was recorded at admission. The accuracy of BS in predicting the need for phototherapy or exchange transfusion treatment was evaluated according to NICE Guidelines for neonatal jaundice management. The bilirubin level obtained by laboratory assay was used as control for treatment prediction.
Results: The prediction of BS to determine need of phototherapy (Table 1A) was accurate in 88% of the cases, with a sensitivity of 77%, specificity of 94%, Positive Predictive Value (PPV) of 87% and Negative Predictive Value (NPV) of 88%. BS was accurate in predicting the need for exchange transfusion therapy (Table 1B) in 99% of the cases, with a sensitivity of 90%, specificity of 99%, PPV of 93% and NPV of 99%.
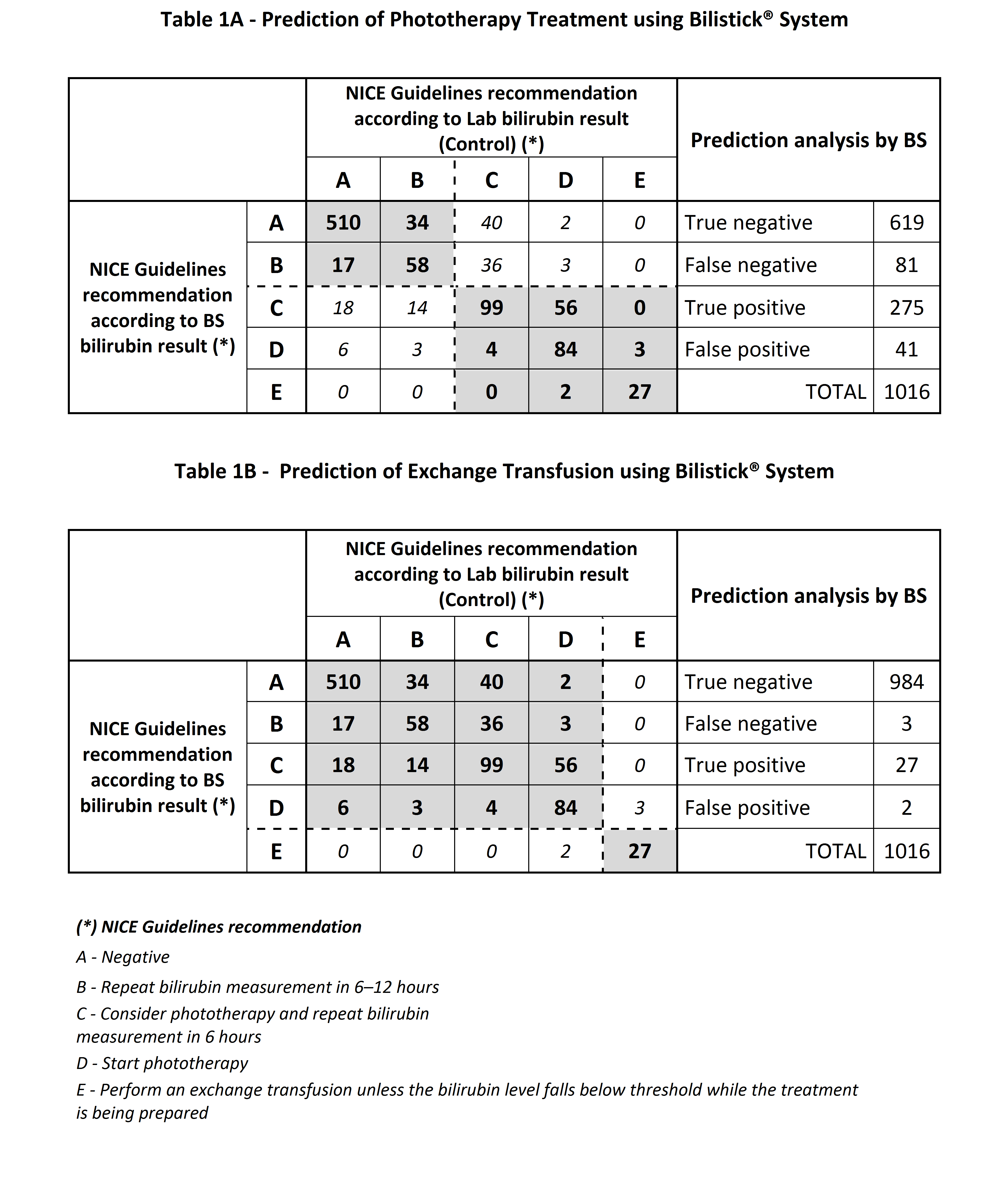
Conclusion: Bilistick® System is a cost-effective POC bilirubin assay able to accurately predict treatment in the case of neonatal hyperbilirubinemia according to NICE Guidelines recommendations.

Powered by Eventact EMS