
Improving Bioethanol Production Process: towards Economically Favorable Pretreatment and Enzymatic Hydrolysis Steps
2Department of Biomolecular Sciences, Weizmann Institute of Science, Rehovot, Israel
Second-generation bioethanol, produced from polysaccharides (mainly cellulose) found in lignocellulosic biomasses, is a promising alternative energy source. Its production process involves enzymatic hydrolysis of the polysaccharides into fermentable sugars, which are then used for alcoholic fermentation. In addition, a pretreatment step is required for improving the enzymatic hydrolysis (i.e., by removing the lignin from the plant cell walls). The relatively high costs of biomass pretreatment and the enzyme production process render bioethanol production economically unfavorable. In order to overcome the techno-economical hurdles, three approaches were explored:
- Biological pretreatment was studied, whereby lignin is mineralizedby the white rot fungus, Pleurotus ostreatus PC9. Several mutant strains were evaluated for their selective lignin mineralization and pretreatment efficiency.
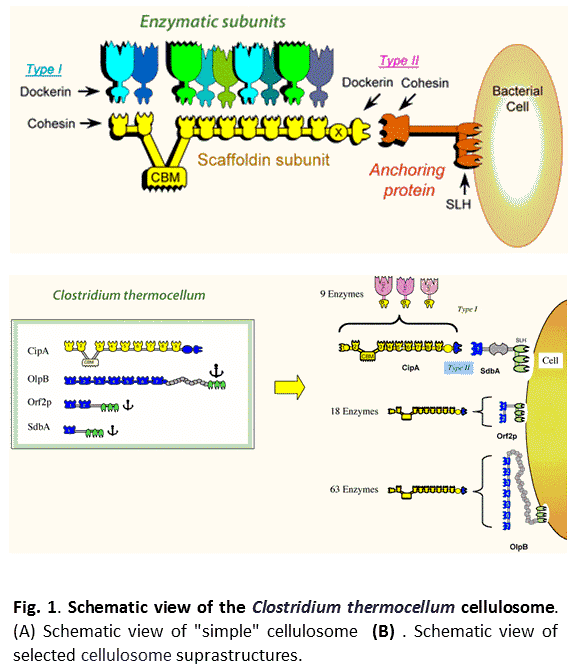
- A diverse set of enzymes is required for an efficient hydrolysis step. An optimized enzymatic cocktail can decrease the enzyme dosage required for hydrolysis without decreasing ethanol yield. In this context, the cellulolytic machinery of Clostridium thermocellum, one of the best-known cellulose-degrading bacteria, was studied. The machinery, called cellulosome, comprises a multi-protein complex that contains a multiplicity of catalytic subunits, as well as structural proteins (Figure 1). The cellulosomal subunit-composition is known to change in response to the carbon source of the medium. Here, six different cellulosome preparations were analyzed. The composition of the superior cellulosomes can indicate key parameters important for efficient lignocellulose biomass hydrolysis.
- The thermostability of thermocellum β-glucosidase A (the last enzyme in the cellulose hydrolysis pathway) was improved, resulting in doubling the glucose yield from the cellulose hydrolysis reaction.
The results reported here can promote economically efficient bioethanol production and provide a "classical" biotechnological case study.
Powered by Eventact EMS