
Fully Integrated Sample-to-Result Microfluidic Cartridges for Point-of-Care Diagnostics
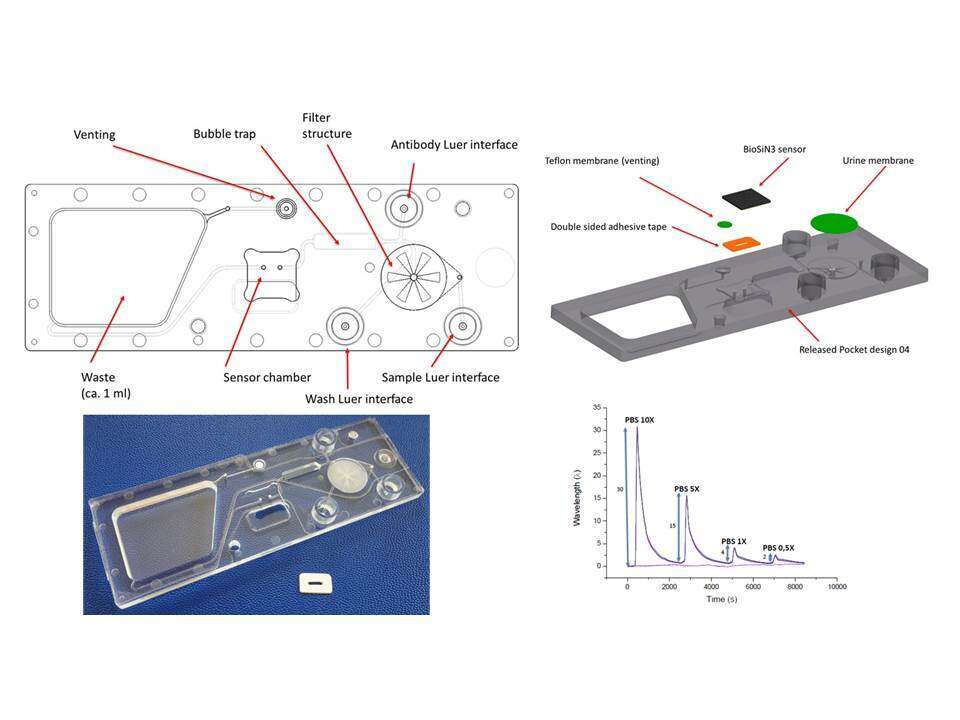
Bringing complex diagnostic assays such as molecular diagnostic protocols to the point-of-care requires the development of highly integrated microfluidic cartridges and respective instruments. These cartridges allow the execution of a diagnostic assay from sample-in to result-out without any user intervention after sample introduction. The technical development of such integrated microfluidic devices however poses a range of specific challenges: Firstly, the complex interaction of the individual functional elements of such a cartridge limits the ability to do a system-level modelling. Secondly, there are no standards comparable to e.g. microelectronics with element libraries. Thirdly, these cartridges are composed from a multitude of different materials, e.g. injection molded polymers, filter membranes, liquid reagent blisters, Si-based sensors and/or metal electrodes, for which manufacturing technologies from different industries have to be employed. In this paper, we present a dedicated development strategy for such integrated microfluidic devices and present a variety of sample-to-result diagnostic cartridges for POC tests, both molecular diagnostics as well as immunological assays in applications such as Tuberculosis, HIV or multiplexed biothreat pathogens. We will highlight specific technological solutions for elements such as on-chip valves, reagent storage and integration of silicon-photonics or electrochemical sensors. We will discuss aspects of cartridge-instrument interfacing with regards to operational robustness and system simplicity as well as selection criteria for optical and non-optical detection technologies based on an overall system architecture.
Powered by Eventact EMS