
Need for Emergent Sternotomy Due to Dynamic Occlusion of Left Ventricular Outflow Tract by an Embolized Watchman Device.
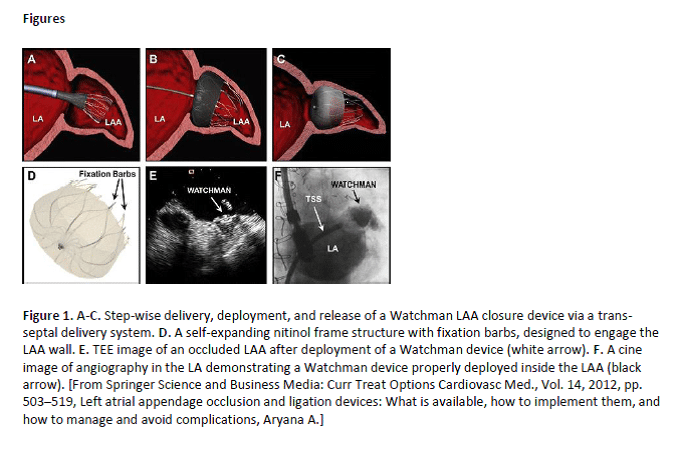
Atrial fibrillation (AF) is the most common arrhythmia seen in clinical practice. Patients with AF are at an increased risk of thromboembolic events, perioperative morbidity, and mortality. The WATCHMAN left atrial appendage (LAA) closure device (Boston Scientific Corp), approved by the FDA in 2015, provides one alternative to chronic systemic anticoagulation for stroke prevention in selected patients. Left atrial appendage closure (LAAC) with this device has been reported to have a high success rate in complete LAAC with low peri-procedural risk, even in a population with a higher risk of stroke and bleeding, and multiple co-morbidities.
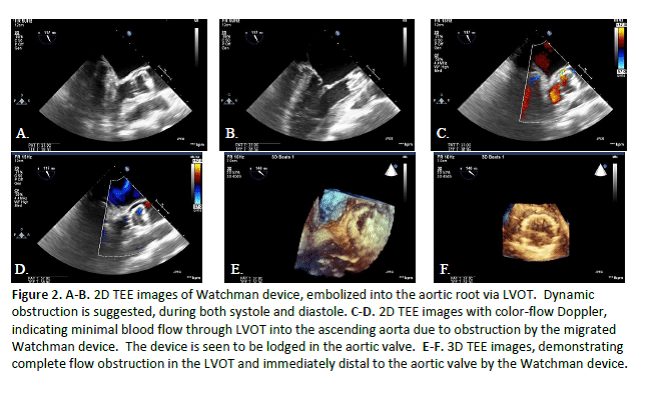
Here we describe a case of a 69 year old male patient, with history of hypertension, hyperlipidemia, insulin-dependent diabetes (DM2), and chronic atrial fibrillation (AF), who underwent WATCHMAN device implantation. In the post-anesthesia care unit, shortly after the procedure, the patient became acutely hemodynamically unstable, and was noted to have device embolization into the left ventricular outflow tract (LVOT) on bedside transesophageal echocardiography. The device was seen dynamically obstructing LVOT, with subsequent significant drop in cardiac output and resultant ventricular fibrillation cardiac arrest. The patient required emergent sternotomy with cardiopulmonary bypass, device retrieval, and surgical LAA closure.


Powered by Eventact EMS