
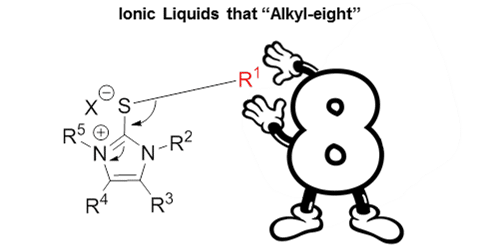
Thioimidazolium Ionic Liquids as Tunable and Versatile Alkylating Agents
Alkylating ionic liquids (AILs) based on the thioimidazolium structure combine the conventional properties of ionic liquids, including low melting point and non-volatility, with the alkylating function. Alkyl transfer occurs exclusively from the S-alkyl position, thus allowing for easy derivatization of the structure without compromising specificity. We apply this feature to tune the electrophilicty of the cation to profoundly affect the reactivity of these AILs, with a “green” caffeine-derived AIL possessing the highest reactivity. Anion choice was found the affect reaction rates, with iodide anions assisting in the alkylation reaction through a “shuttling” effect. The ability to tune the properties of an alkylating agent simply using the toolbox of ionic liquid chemistry highlights the modular nature of AILs as a platform for alkylating agent design and integration in to future systems.
Powered by Eventact EMS