
Milk Protein Based Nanovehicles for Oral Delivery of Chemotherapeutic Combinations to Overcome Multidrug Resistance in Gastric Cancer
2Laboratory of Food Physical Chemistry and Biopolymeric Delivery Systems for Health, Department of Biotechnology and Food Engineering, Technion-Israel Institute of Technology, Haifa, Israel
3The Fred Wyszkowski Cancer Research Laboratory, Department of Biology, Technion-Israel Institute of Technology, Haifa, Israel
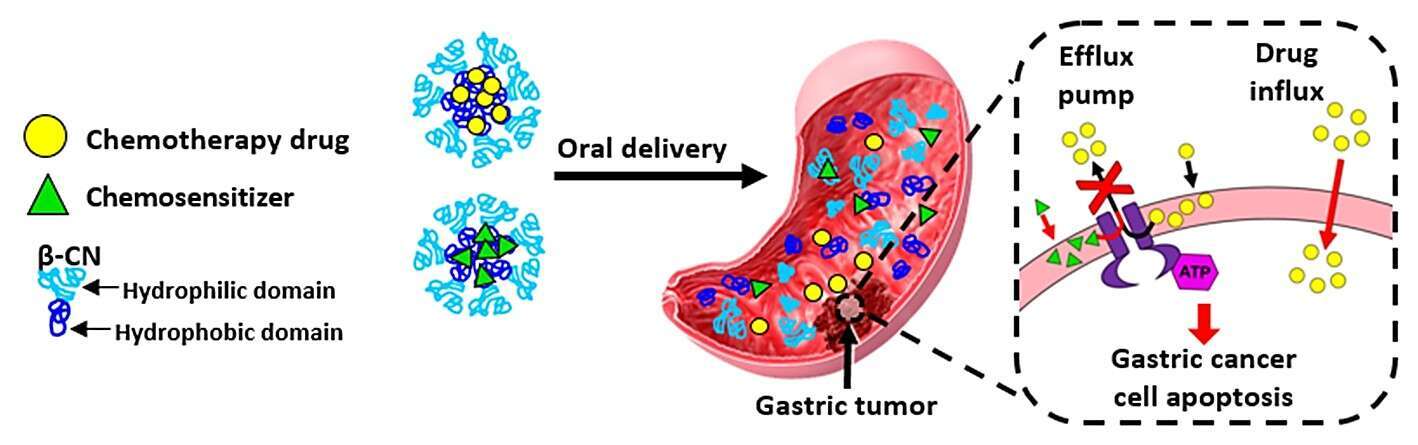
Gastrointestinal cancers are the third leading cause of cancer-related mortality worldwide. Moreover, cancer multidrug resistance (MDR) remains a primary hindrance toward curative therapy. Furthermore, most chemotherapeutic agents are lipid-soluble, administered intravenously using harmful solvents and surfactants. An effective and selective oral delivery system would significantly contribute to patients’ quality of life, reduce hospitalization costs and circumvent infection with antibiotic-resistant pathogens widespread in hospitals. Bovine β‑casein (β‑CN) is an abundant milk protein that has a prominent amphiphilic structure, promoting it`s self-assembly to stable micelles in aqueous solutions. In previous studies we demonstrated the potential of β‑CN micelles (β‑CM) to serve as nanovehicles for oral delivery and target-activated release of hydrophobic drug cargo in the stomach. Herein we introduce an oral delivery platform based on β‑CM, to deliver a synergistic drug combination of a chemotherapeutic drug along with its corresponding chemosensitizer, which counteract ATP-driven MDR efflux pumps (e.g. p-glycoprotein (P-gp)/ ABCB1, breast cancer resistance protein/ ABCG2), that expel a spectrum of anticancer drugs from cancer cells and therefore markedly suppresses the efficacy of numerous hydrophobic chemotherapeutics. Hence, the rationally designed encapsulated pair is expected to display enhanced efficacy and synergy in overcoming MDR phenomena in gastric cancer. This novel treatment strategy, can allow less painful treatment at the comfort of the patient`s home. The target-activated release mechanism aimed to locally treat gastric cancer can diminish untoward toxicity to the upper gastro-intestinal tract and minimize toxic side effects caused by systemic chemotherapy. The suggested nanosystem could also be applied for the treatment of various other gastric disorders and tailored to individual drug combinations for personalized medicine.
Powered by Eventact EMS