LIPASE CATALYZED,IN-SITUGENERATION OF HYPERVALENT IODINE REAGENT FOR SELECTIVE ALCOHOL OXIDATION
Hypervalent iodine reagents are a powerful class of oxidation reagents, which have earned their popularity to their highly selective character.[1] On the other hand their atom-efficiency is extremely low, due to the stoichiometric co-use of iodine. The selective character of hypervalent iodine in alcohol oxidation is noteworthy. In highly functionalized alcohols, such as steroids, fast and reliable oxidation of the alcoholic function to the ketone is observed.[2] This selectivity is unsurpassed by common catalytic methodologies.[3]
In the current paper we describe a biocatalytic approach towards the in-situ generation of these iodine reagents. Only recently, the catalytic use of iodine reagents in oxidation has been published by different authors [4]. However in all these approaches peracids and oxone are used as stoichiometric oxidants. In our approach hydrogen peroxide is used as the final oxidant, to achieve highly selective oxidation of functionalized alcohols to the corresponding aldehydes and ketones. The basis for this methodology is a three-step-reaction as described in Scheme 1.The iodine source is Iodine(I) functionalized polystyrene. The strength of this methodology will be in the oxidation of alcohols that can not be oxidized by currently available, metal-based, catalytic oxidation methods. Therefore, this Iodine(I) cascade using hydrogen peroxide as oxidant was specifically tested for the oxidation of steroidal alcohols. 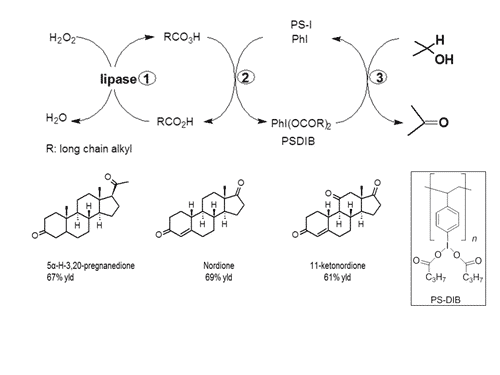
References
1. T. Wirth, (Ed.) Top. Curr. Chem., 2003, 224.
2. A. Kotlewska, Thesis, Delft University, 2010.
4. (a) T. Dohi, Y. Kita, Chem. Commun., 2009, 2073 (b) R. Mu et al. Adv. Synth. Catal., 2005, 347, 1333. (c) C. I. Herrerias, T. Y. Zhang, C-J. Li, Tetrahedron Lett., 2006, 47, 13.
Powered by Eventact EMS