
Controlling the Secondary Sphere of Organocatalysts by Orthogonal Directing Groups
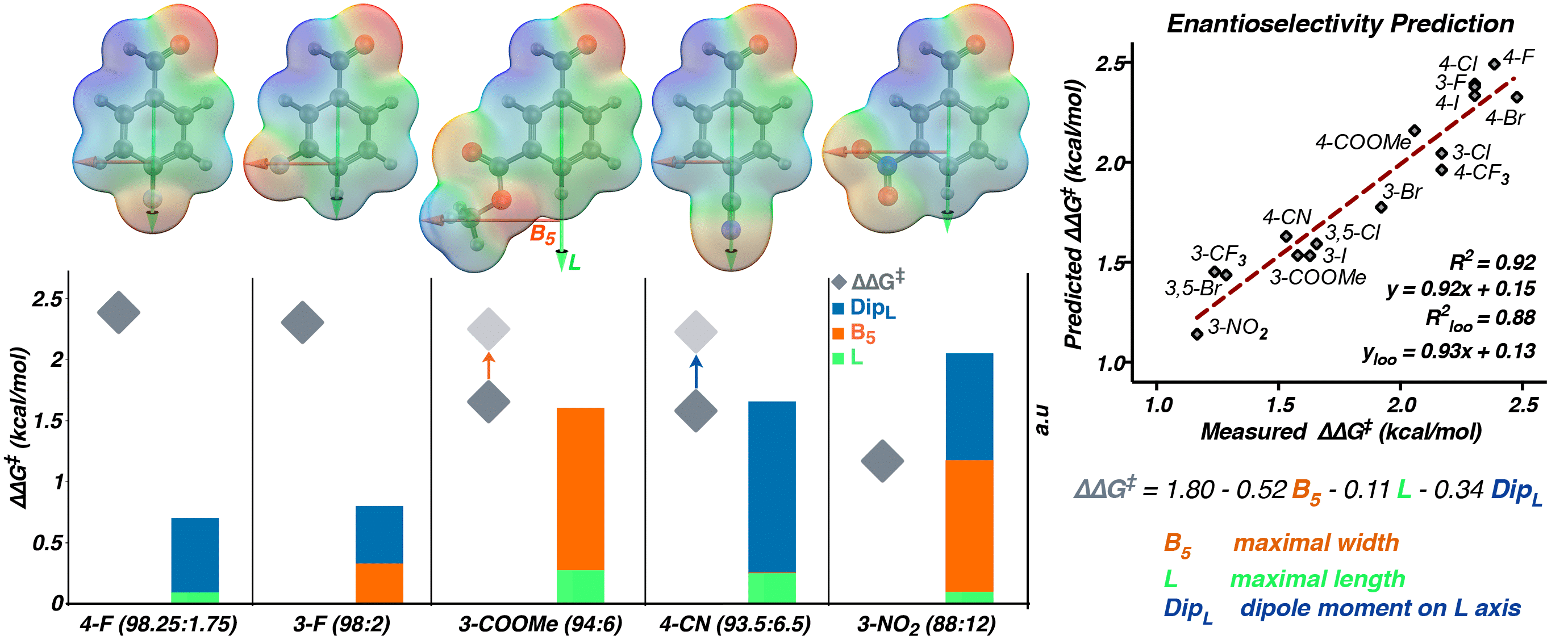
Inspired by the fundamental effect of secondary-sphere non-covalent interactions on enzymatic catalysis, metal-mediated processes and transition-metal chemistry, our group seeks to offer an approach for the facile in-situ modification and mechanistic interrogation of secondary-sphere interactions in organocatalysis. As a proof-of-concept, we have modified the secondary-shpere of N-heterocyclic carbenes (NHCs) using boronic acids (BAs) in-situ under reaction conditions and tested their reactivity and selectivity in the benzoin reaction. This modification leads to unprecedented enantioselectivity, even with challenging electron-withdrawing substrates. Furthermore, in contrast to the results obtained with the NHC catalyst alone, the enantioselectivity is highly reproducible under our reaction conditions. We applied mathematical modelling techniques to uncover the structural origin of the enantioselectivity in this system, suggesting two possible reasons for its erosion in certain cases. This strategy allowed us to propose two different methods to improve selectivity. Our mechanistic studies indicate that BAs play a dual role—increasing the reactions’ enantioselectivity and also inhibiting the racemization of the benzoin product.
Powered by Eventact EMS