
Mechanistic Oriented Approach Towards Oxidative Cross-Coupling Reactions of Phenols by Iron Catalysis
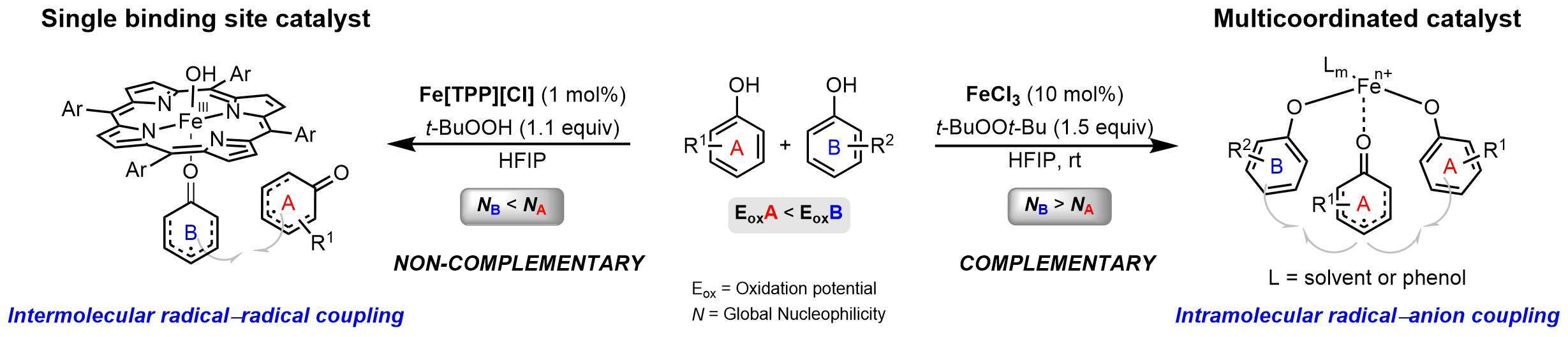
Metal catalyzed oxidative coupling of phenols is a powerful method for preparing complex phenol-based materials by simple and sustainable means. As part of our group’s interest to develop mechanistic-oriented oxidative coupling reactions, a set of catalytic systems that ensure complete control over the chemoselectivity regardless the electronic nature of the phenolic coupling partners is under development.
Our study revealed that oxidative cross-coupling of phenols mediated by a multi-coordinated FeCl3 catalyst proceeds via a chelated radical−anion coupling mechanism.1 Based on mechanistic studies, electrochemical methods and density functional theory (DFT) calculations, a general model was established to predict the feasibility of the cross-coupling for a given pair of phenols. The catalytic system was proved to be highly chemoselective for phenols that maintain complementary relationship (Scheme 1) but less efficient for noncomplementary phenolic coupling partners. Due to this mechanistic limitation, an alternative mechanistic route that involves coupling of readily oxidized phenols with poor nucleophilic phenolic partners has been developed. The novel catalytic system is based on Fe[TPP]Cl complex, which mediates outersphere coupling between a liberated phenoxyl radical and an iron-ligated phenolic coupling partner.2
(1) Libman, A.; Shalit, H.; Vainer, Y.; Narute, S.; Kozuch, S.; Pappo, D. J. Am. Chem. Soc. 2015, 137, 11453-11460.
(2) Shalit, H.; Libman, A.; Pappo, D. J. Am. Chem. Soc. 2017.
Powered by Eventact EMS