
Keynote
Can we Synthesize Life in the Lab? How Chemistry may become Biology
How the immense complexity of living organisms has arisen is one of the most intriguing questions in contemporary science. We have started to explore experimentally how organization and function can emerge from complex molecular networks in aqueous solution [1]. We focus on networks of molecules that can interconvert, to give mixtures that can change their composition in response to external or internal stimuli. Molecular recognition between molecules in such mixtures leads to their mutual stabilization, which drives the synthesis of more of the privileged structures (Figure 1). Intriguingly, in this process the assembling molecules are replicating themselves, where replication is driven by self-recognition of these molecules in the dynamic network [2]. The selection rules that dictate which (if any) replicator will emerge from such networks are starting to become clear [3]. We have observed that factors such as mechanical energy [2] and the nature of the environment [4] can determine which replicator wins the competition for building blocks. We have also witnessed spontaneous differentiation (a process akin to speciation as it occurs in biology) in a system made from a mixture of two building blocks [5]. The prospect of Darwinian evolution of purely synthetic molecules is tantalizingly close and the prospect of synthesizing life de-novo is becoming increasingly realistic.
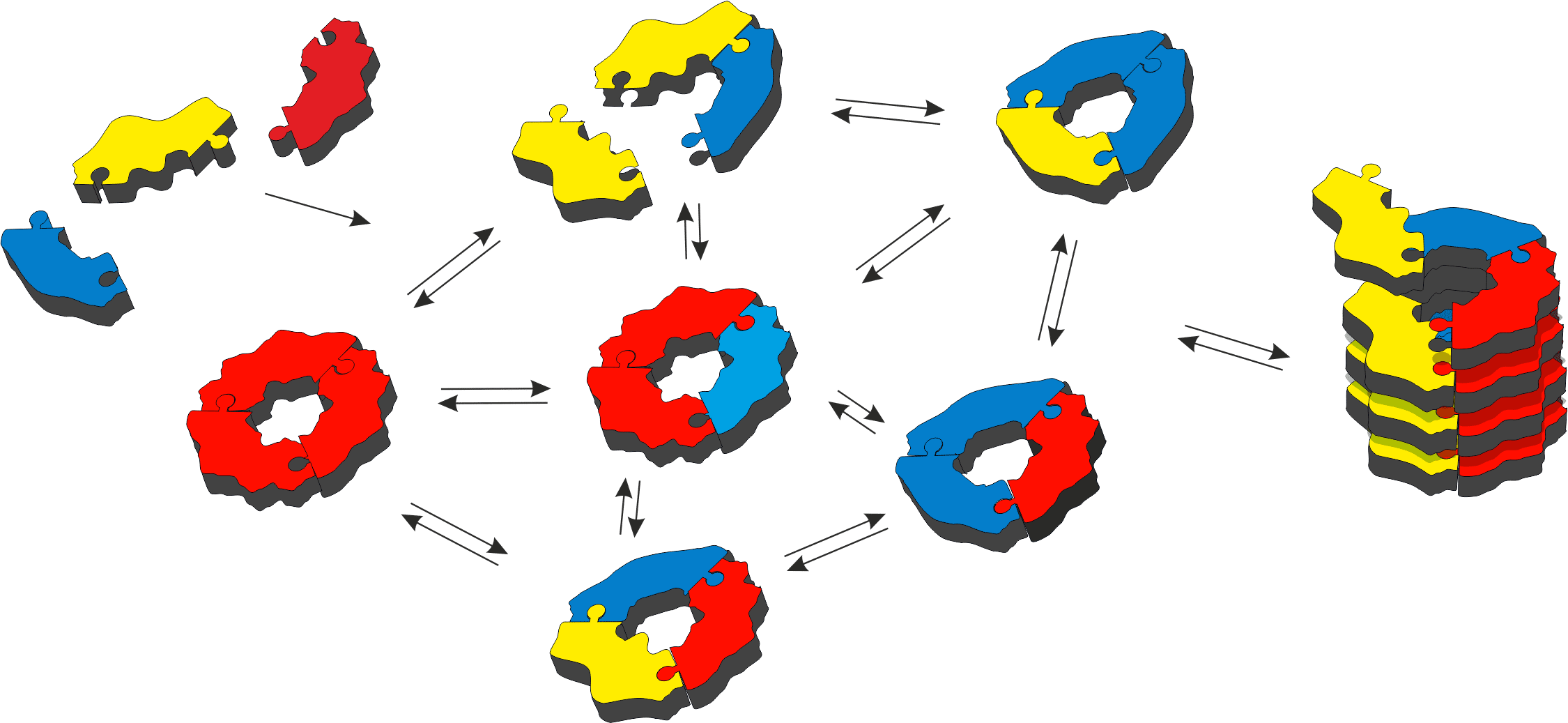
Figure 1 – Molecular recognition between molecules in a dynamic molecular network can lead to self-synthesizing materials, build up from self-replicating molecules.
[1] Li, J.; Nowak, P.; Otto, S. J. Am. Chem. Soc. 2013, 135, 25, 9222-9239. [2] Carnall, J. M. A.; Waudby, C. A.; Belenguer, A. M.; Stuart, M. C. A.; Peyralans, J. J.-P.; Otto, S. Science 2010, 327, 1502-1506. [3] Malakoutikhah, M.; Peyralans, J.J-P.; Colomb-Delsuc, M.; Fanlo-Virgos, H.; Stuart, M. C. A.; Otto, S. J. Am. Chem. Soc. 2013, 135, 49, 18406-18417. [4] Leonetti, G. Otto, S. J. Am. Chem. Soc. 2015, 137, 2067–2072. [5] J. W. Sadownik, E. Mattia, P. Nowak, S. Otto, Nature Chem. 2016, 8, 264–269.
Powered by Eventact EMS