
Electrophoretic Deposition of Single-source Precursors as a General Approach for the Formation of Hybrid Nanorod Array Heterostructures
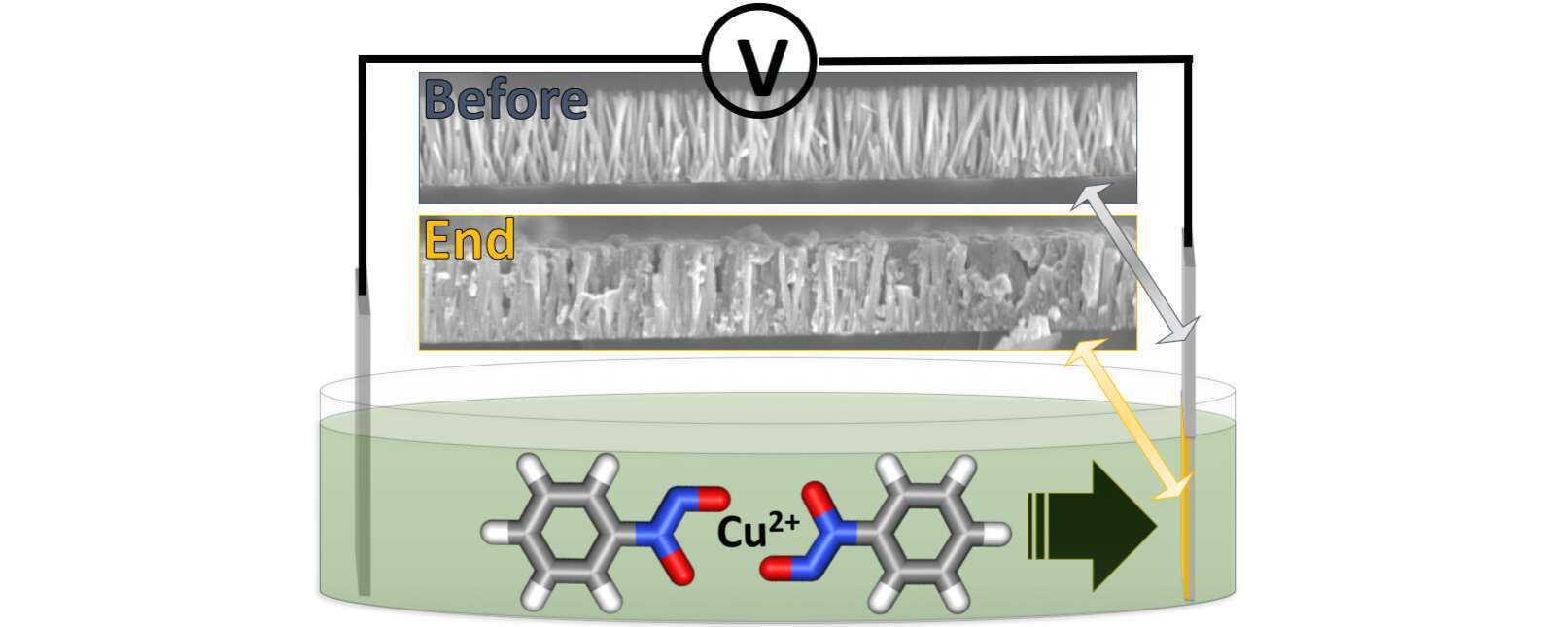
Pyrolysis of single-source precursors (SSP) is a common procedure in the synthesis of different thin films and nanostructures. The most common approaches for the thermal decomposition of SSPs are the gas-phase reaction and colloidal synthesis in high-boiling point organic solvent. The gas-phase is usually used for deposition of thin films, while the colloidal approach is used for the synthesis of various nanostructures and complicated interfaces between colloidal nanoparticles. We investigate electrophoretic deposition (EPD) of SSPs onto conductive substrates covered with ZnO nanorods, followed by thermal decomposition, to achieve ZnO–CuO and ZnO–CdS heterostructures on the nanoscale.
To the best of our knowledge, this is the first time that SSPs are subjected to EPD. This approach allows formation of both thin-films, and the challenging filling and coating of vertically aligned ZnO nanorod arrays as a case-study example for easily scalable nano-heterostructure synthesis. We demonstrate that the use of different SSP families result in metal-oxide and metal-sulfide semiconductors.
This work explores the effect of changing the dispersant medium of the SSP on the final morphology of the formed interfaces. We show that changing the organic dispersant can form both complete filling of the nanorod array and core–shell structures.
The application of the electrophoretic effect, which usually describes colloids subjected to DC electric field, on both dispersed and completely solvated SSPs raises basic scientific questions (what is the nature of the effect of electric fields on metallo-organic molecules, i.e., interplay between electrokinetic phenomena and SSP-solvent interaction) and has high applied potential in the synthesis of thin films and nanostructures.
Powered by Eventact EMS