
Towards Mechanically Interlocked [3]Catenane-Based Polymers
2Department of Chemistry, Ben-Gurion University of the Negev, Beer-Sheva, Israel
Polycatenanes, in particular poly[3]catenanes, are structurally appealing polymers owing to their high degree of mobility and prone to induce unusual and potentially useful improvements of properties compared to classic covalently linked polymers.1 The emergence of novel properties, such as large loss modulus, viscous flow at low activation energy and rapid stress relaxation, are expected if [3]catenane units could be incorporated into covalently linked polymer structures. It is anticipated that employing flexible catenane linkages within the polymer chain may result in a dynamic assembly, significantly altering thermal dissipation (by non-directional rotation “pirouetting”), rheology and/or other mechanical (elasticity) properties.
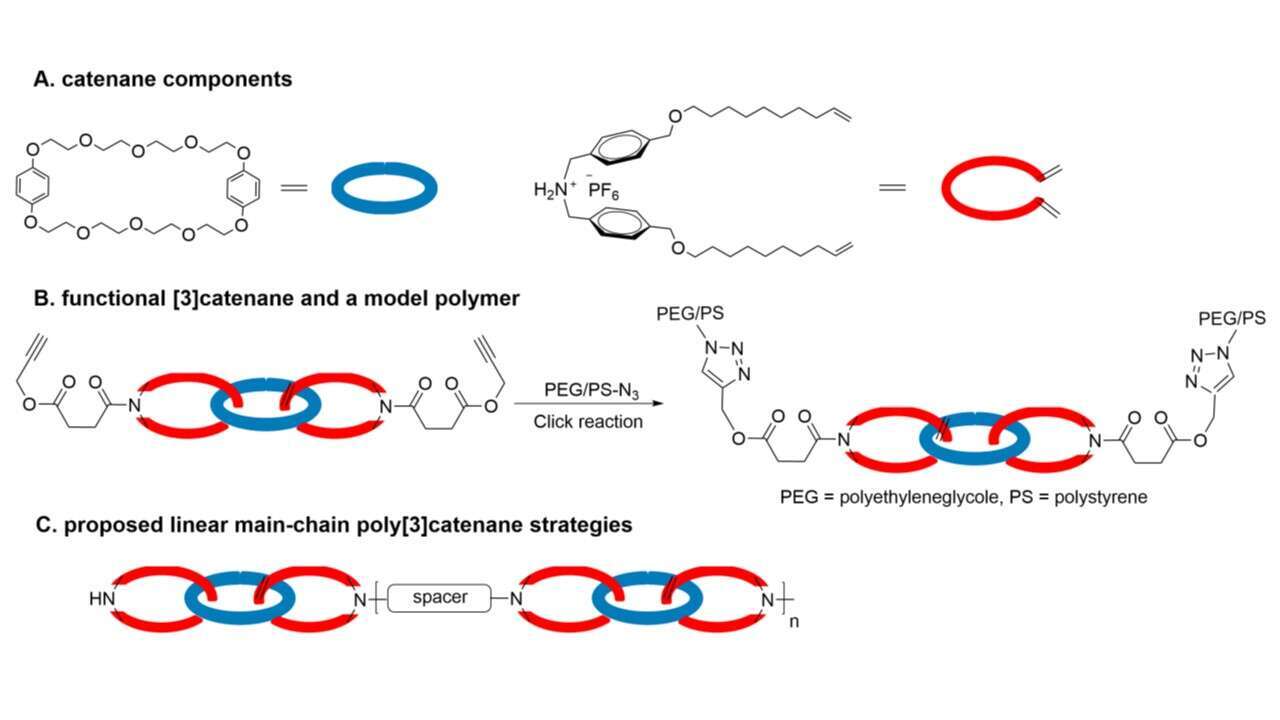
We have devised a synthetic platform to produce a family of functional [3]catenanes and use them to prepare linear main chain poly[3]catenanes. Here we present our preliminary results and describe the challenging synthetic efforts towards our goal. Initially, we synthesized [2]catenane by three steps: (i) threading ammonium salt containing aliphatic long chains equipped with terminal double bonds through crown-ether (A), (ii) RCM2 and (iii) reduction of the double bond. Next, we prepared the desired [3]catenane by repeating the two first steps (i-ii). Finally, attachment of functional pendant arms such as propargyl (B) or hydroxy, to the amino groups of both terminal [3]catenane rings is used for the synthesis of copolymers by azide-yne click reaction and isocyanate condensation. Polymerization of theses monomers is currently underway (C).
References:
- Z. Niu and H. W. Gibson Chem. Rev. 2009, 109, 6024
- H. Iwamoto, S. Tafuku,Y. Sato, W. Akizawa, W.Katagiri, E. Tayama, E. Haegwa, Y. Fukazawa, T. Haino, Chem Commun. 2016, 52, 319
Powered by Eventact EMS