
Synthesis of a Stable Complexed Stannylene [(R3Si)2Sn:]
Much attention has been focused on the preparation and reactivity of tetrylenes, divalent group-14 elements R2E: (E= Si, Ge, Sn). In this work we focus on stannylenes which are important reagents in organic synthesis. There are two common methods for their preparation: (a) substitution reaction of SnCl2 with organometallic species; (b) reduction of the corresponding dihalides, R2SnX2. The major drawbacks of both methods are the relatively low yields of the desired stannylene and the necessity to separate it from salts.
Recently, we found that intermediate bis(silyl)stannylenes can be generated conveniently under mild conditions from trisilyl - substituted tin hydrides (Scheme, step a) and can be trapped by several reagents. In the case of (tBu2MeSi)3SnH (1), the intermediate bis(silyl)stannylene (2) dimerizes instantaneously yielding distannene (4) (Scheme, step b). However, when 2 is generated in the presence of NHCMe, quantitative conversion to carbene-stannylene complex (3) is obtained (Scheme, path c). In contrast to free stannylene 2, NHCMe complexed stannylene 3 does not dimerize.
In recent years, the chemistry of R2E: coordinated by NHC attracts wide interest. Now, that we have both the uncomplexed stannylene 2 and the NHCMe complexed stannylene 3, we plan to compare their reactivities with various compounds.
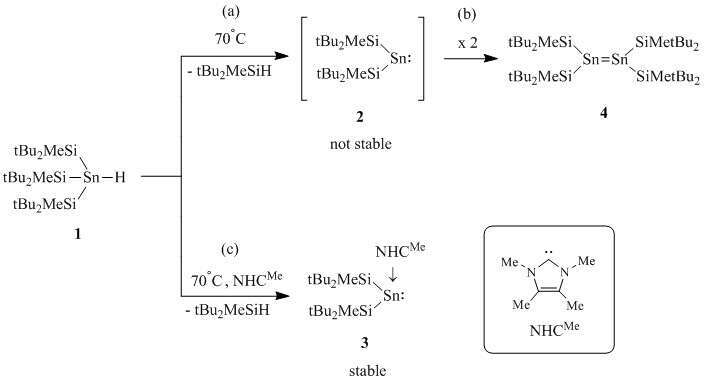
Scheme
Powered by Eventact EMS