
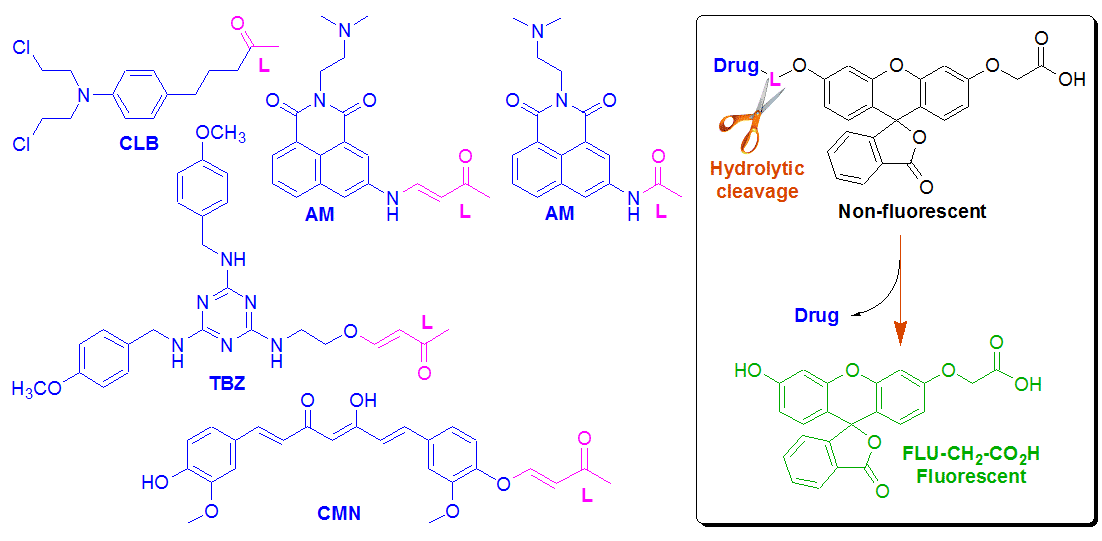
Novel Fluorescein Based Drug Conjugates for Fluorescent Monitoring in Drug Delivery
The fluorescence based sensing of drugs transported to specific biological targets has emerged as an important research topic in the field of drug delivery monitoring. In this work, bifunctional, on-off switchable fluorescein reporter FLU-CH2-CO2H was synthesized and bound to anticancer drugs — chlorambucil (CLB), tubulizine (TBZ), curcumin (CMN), and amonafide (AM) by means of different biodegradable linkers (L). The drug release triggered by bio-hydrolytic environment was fluorescently monitored and the obtained cleavage profiles were found to be in good agreement with those measured by spectrophotometric and LC/MS methods. The cleavage rates are strongly dependent on the linker nature. The half-lives vary in a wide range between 20 sec and 14 h. Calibration curves for the fluorescence signal versus the released drug concentrations were obtained.
|
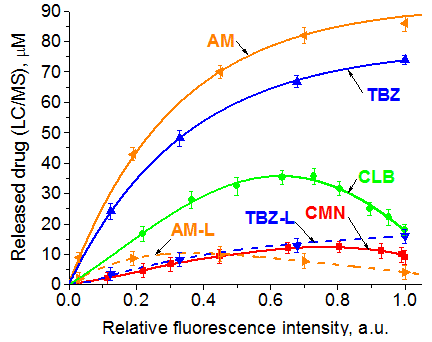
Figure on the right represents the calibration curves showing the free drug (solid line) and Drug-L residue (AM-L and TBZ-L) concentrations (dashed line) vs. the FLU-CH2-CO2H relative fluorescence intensity signal measured at 516 nm in time dependent mode for each conjugate. Time intervals were estimated and cleavage experiments were performed in cell medium (37 oC) for the initial conjugate concentrations 90 mM. The developed fluorescein based drug conjugates containing aromatic and aliphatic oxy- and aminoacrylate linkers are considered a versatile fluorescent platform for monitoring of drug delivery in vivo. |
 |
We suggest that these conjugates can be useful for binding to the receptor specific ligands through a free carboxylic group so that their targeted hydrolysis inside living cells will be monitored by fluorescence microscopy and flow cytometry.
Powered by Eventact EMS