
Cyanine Dyes for Fluorescence Based Monitoring in Targeting Drug Delivery
Basic targeting drug delivery platforms comprise a drug attached to a targeting carrier for recognizing the abnormal cells. Tagging of these platforms with a fluorescent dye enables monitoring of targeting drug delivery (TDD) and thereby facilitates personalized medicine and allows disease-targeting treatments, including for cancer patients. In this work, we develop synthetic approach and synthesize novel fluorescent pentamethine cyanine dyes of unsymmetrical structure and explore these dyes for fluorescent labeling of anticancer drug chlorambucil (CLB). These new dyes contain two different reactive functionalities selected from groups CO2H, NH2 and OH. One of these groups (NH2 and OH) was utilized for binding to CLB carboxylic group by means of a biodegradable linker, while another one can be further used for binding to a cell-specific targeting carrier (peptide or antibody).
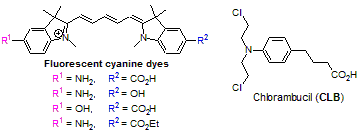
We investigated spectral properties of these new dyes including the absorption and fluorescence spectra and quantum yields. The dyes absorb and emit within the long-wavelength spectral region (600–660 nm) enabling fluorescent monitoring in deeper tissues. Furthermore, we attached these dyes to CLB and investigated the drug cleavage profiles in hydrolytic environment that simulate biological conditions in target cells. The cleavage profiles were measured by LCMS, spectrophotometrical and spectrofluorometrical methods. Effect of biodegradable linker on the CLB cleavage rates was studied.
Due to their long-wavelength excitation and emission, reasonable brightness and stability, the new dyes are suitable for monitoring of drug transport and drug delivery to target cells
Powered by Eventact EMS