
The Fate of Methyl Radicals in the Radiolysis of Aqueous DMSO- Revisited
2Chemistry Department, Nuclear Research Centre Negev, Be'er Sheva, Israel
3Chemical Sciences Department and the Schlesinger Family Center for Compact Accelerators, Radiation Sources and Applications, Ariel University, Ariel, Israel
Reactions of DMSO in aqueous solutions under continuous radiolysis have been studied and used to produce a steady state concentration of methyl radicals. Using pulse radiolysis, the reactions of DMSO at a high dose rate were studied as well.
Our study focuses on the reactions which occur in solutions of aqueous DMSO at a low steady state concentration of •CH3.
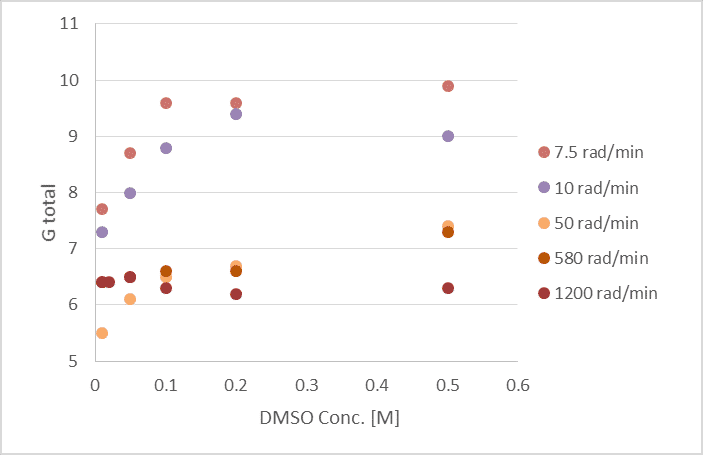
-Experiments using different DMSO concentrations and multiple dose rates have been performed.
-Experiments including a mixture of DMSO and DMSO-d6 have been conducted, and the GCMS data of the aqueous phase products has been analyzed.
The results show an increase in total G and in the methane/ethane ratio as a function of DMSO concentration with a strong dependence on the dose rate.
Calculating the steady state concentration of •CH3, one can show that at low dose rates, the kinetic equations are no longer compatible with the measured system, and points out the idea that most of the ethane produced does not come from the biradical termination reaction of two methyl radicals.
The dependence of the obtained results on the concentration of DMSO points out a possible 2-step reaction mechanism involving DMSO and methyl radicals.
Results also point out •CH3S(O)CH2 as a reactive radical which can produce more •CH3 radicals and form a DMSO ‘dimer’ as a side-product.
Detailed data and mechanisms will be presented.
Powered by Eventact EMS