
N-Heterocyclic Carbene Boronic Esters (NHC-BE) for the Formation of Electron-Withdrawing Benzoin Products
The benzoin reaction has been widely investigated in the field of organocatalysis. This reaction was originally discovered by Wöhler and Liebig in 1832 using cyanide as a catalyst.1 In recent years, numerous research groups have focused on developing enantioselective routes to form benzoin products using N-Heterocyclic Carbene (NHC) catalysts in the presence of an appropriate base.2
In 2009 Zeitler, Connon and colleagues reported the most efficient conditions for the enantioselective benzoin reaction to date using catalytic amount of pentafluorophenyl-substituted NHC (4 mol %) and Rb2CO3 in THF (1.1M) at 0 oC to room temperature.3 However, to date, the enantioselective benzoin reaction has not been extensively studied with electron-withdrawing substituted aryl-aldehydes. Herein, we wish to demonstrate our new concept for enantioselective benzoin reaction catalyzed by NHC (6 mol%) in the presence of Rb2CO3 (4 mol%), MS, and boronic acid (7.2 mol%) in dry THF (1.1M) at 20 oC.4 These new conditions afforded highly enantioselective benzoin reactions for electron-withdrawing substrates (F, Cl, Br, I, CN, CO2Me, CF3 and NO2) in the range of 86-97 %ee with good yields.
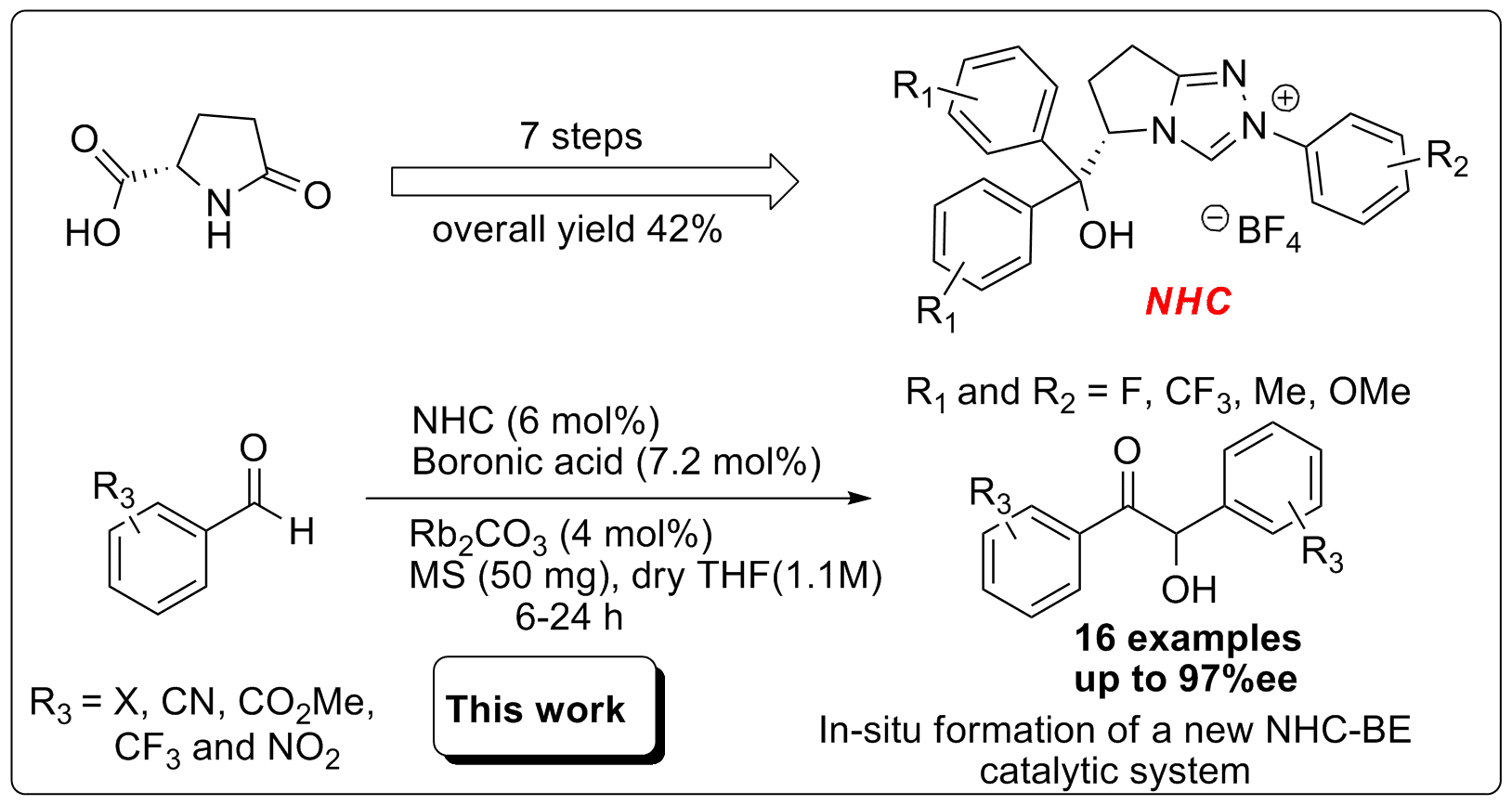
Figure 1. Enantioselective benzoin reaction catalyzed by NHC-BE
References
- Wöhler, F.; Liebig, J. Ann. Der. Pharm. 1832, 3, 249-287.
- Flanigan, D. M.; Michailidis, F-R.; White, N. A.; Rovis, T. Chem. Rev. 2015, 115, 9307-9387.
- Baragwanath, L.; Rose, C. A.; Zeitler, K.; Connon, S. J. J. Org. Chem. 2009, 74, 9214-9217.
- Neel, A. J.; Milo, A.; Sigman, M. S.; Toste, F. D. J. Am. Chem. Soc. 2016, 138, 3863-3875.
Powered by Eventact EMS