
Chemical Modification of Natural QS Autoinducers to Unravel the P. aeruginosa QS Regulon
In recent years the world has seen a troubling increase in antibiotic resistance among many kinds of bacteria, especially in hospitals. Certain pathogen bacteria have gained resistance against most known antibiotics. An alternative approach of treating and / or preventing bacterial infections is to target the bacterial communication mechanisms that enable bacteria to collaborate with one another. Many bacterial species use a form of cellular communication in order to coordinate gene expression and this system is termed "Quorum Sensing" (QS). This type of cell to cell communication helps the bacteria to sense population density and express genes in a collective manner. In order to sense their `quorum`, the bacteria use small molecules called autoinducers. These molecules are synthesized by the bacteria and secreted into the growth medium, thus function as a bacterial "language". This way bacteria assess their population density and regulate virulence accordingly. Our aim is to attain a deeper molecular understanding of these processes through synthesis of chemically modified autoinducers.
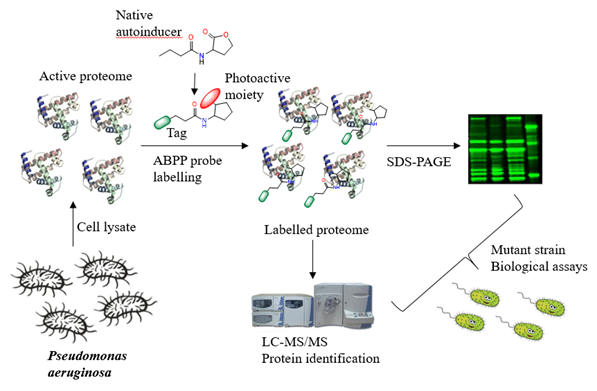
In Pseudomonas aeruginosa one of the native autoinducers is N-butyryl-L-homoserine lactone (C4-HSL). Using an activity-based protein profiling (ABPP) strategy we designed and modified the basic structure of this native autoinducer and introduced a photoreactive group and a ‘click’ tag as an analytical handle. With these probes we have been able to visualize, separate and identify previously uncharacterized proteins that are part of the P. aeruginosa QS network. By further examination with knockout bacterial strains, we found a connection that may uncover another role for this natural autoinducer in the Rhl QS system in P. aeruginosa.
Powered by Eventact EMS