
Metal-Ligand Cooperation in Pd-PNO Pincer Complexes
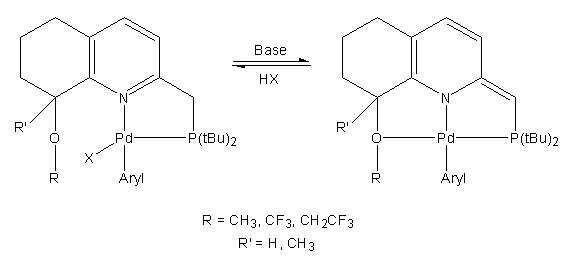
Since their discovery in the mid 1970’s, late-transition metal pincer complexes have been a rich source for new chemical reactions. More recently, these complexes were utilized for catalytic transformations via the metal-ligand cooperation (MLC) mechanism. A key process in these reactions is the reversible aromatization-dearomatization of the pincer ligand. Our group was able to expand the scope of MLC to cross-coupling chemistry and develop a series of novel E-H (E =C, N, O, S) bond activation-based catalytic transformations. While much effort was invested into the design of such transformations, little is known about the application of reversible dearomatization in creating new C-E (E= C, N, O) bonds via the reductive elimination mechanism.
In this study, we report the design and synthesis of several novel bidentate “quasi-pincer” metal complexes based on a PNO-type ligand scaffold. The ligands are derived from a quinoline core substituted with different “reluctant” alkoxy side arms. The use of a “reluctant” side arm can provide the possibility of a temporary dissociation of the donor, which in turn enables creation of a vacant coordination site. Thus, allowing for substrate activation at the metal center.
We hypothesized that the electron withdrawing effect of the side arm can affect the metal-binding lability. We have examined several ligands designs bearing different directing functional groups with the purpose of unprecedented C-E cross-coupling reactions (E = C, N, O) through the metal-ligand cooperation mechanism.
The results of this study demonstrate that the quinoline core bearing the alkoxy side-arm palladium (ll) complexes has the propensity to catalyze cross-coupling reactions, under the relatively unexplored metal-ligand cooperation mechanism, for cross-coupling chemistry. Our current efforts towards the discovery of new reaction mechanisms involve structural modification at key positions on the pincer ligands.
Powered by Eventact EMS