
Dendronized Cellulose Made by Covalent Attachment of Modular Building Blocks and Thiol-ene Click Chemistry
2Department of Organic Chemistry, School of Chemistry, Faculty of Exact Sciences, Tel-Aviv University, Tel Aviv, Israel
3Center for Nanoscience and Nanotechnology, Tel-Aviv University, Tel Aviv, Israel
4Department of Physical Chemistry, School of Chemistry, Faculty of Exact Sciences, Tel-Aviv University, Tel Aviv, Israel
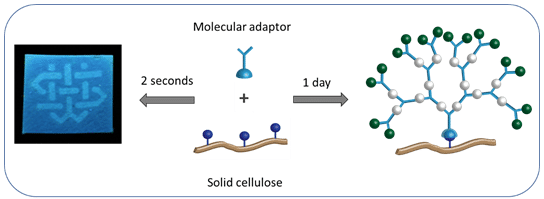
We present a technically simple and general method for covalent modification of solid cellulose with dendrons, as a platform for grafting with functional materials. Surface functionalization of cellulose with first generation dendrons was achieved by esterification with bifunctional molecular adaptors based on 2,2-bis(hydroxymethyl) propionic acid (bis-MPA) under mild conditions and short reaction times. The adaptor-activated surface displayed hydrophobic properties and contained two reactive alkene end-groups per graft. These end-groups were further used for covalent binding to active agents, as demonstrated by selective functionalization of the modified cellulose with fluorescent dye using photopatterning. In addition, the number of active end-groups on the surface of cellulose was multiplied using divergent solid-state synthesis of second and third generation dendrons having four and eight reactive sites per dendron, respectively. The dendrons were assembled in only few hours using the molecular adaptor as a branching unit and a sequence of thiol-ene/esterification reactions. The accurate control of the number of binding sites on the surface of cellulose allowed fine tuning of the surface properties, as demonstrated by the attachment of hydrophobic small molecules to the dendronized cellulose. The ability to produce well-defined and chemically reactive dendron architectures on the surface of solid cellulose is significant in light of the numerous advanced applications that utilize functional cellulose surfaces.
Powered by Eventact EMS