
Participation of Elderly in Heart Failure Randomized Clinical Trials Throughout the Last Decade
Introduction: Heart failure (HF) is major cause for morbidity and mortality worldwide. Although both the incidence and prevalence of HF increases with age, the reported representation of elderly in clinical trials is low. We explored the degree of participation of elderly in contemporary clinical trials published during the last decade.
Methods: MEDLINE database was systematically searched for randomized controlled trials (RCT) published between November 2007 and November 2017 and indexed under subject heading “heart failure”. Following the PRISMA consensus, 1179 studies were identified. After screening and excluding of irrelevant studies and those reporting
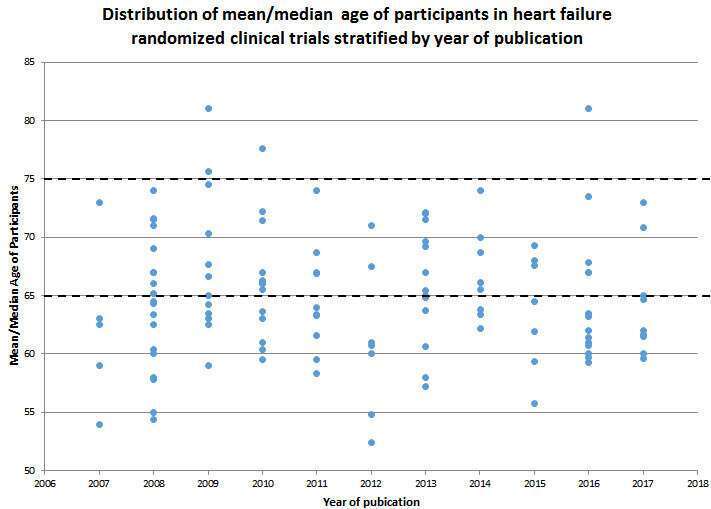
Results: The included studies enrolled 130,561 patients. Drug effect was explored in 44% of the studies, device/procedure in 19% and the rest were lifestyle modification, monitoring and other therapeutic interventions. The mean age of participants varied significantly between the studies (figure) with no notable trend for increase in participation of patients older ≥65 years. A mean age of participants ≥65 years was recorded in only 47% of the studies, while in 3% of studies, the mean age was ≥75 years.
Conclusion: Less than half of HF RCT’s published in the last decade included patients with mean age ≥65 year. As the population ages, it is imperative for trial designers to include a substantial number of elderly patients in order to establish the merits of interventions in this growing population.
Powered by Eventact EMS