
CRISPR-Targeted Genome Editing of Human Mesenchymal Stem Cells for Infarct Repair
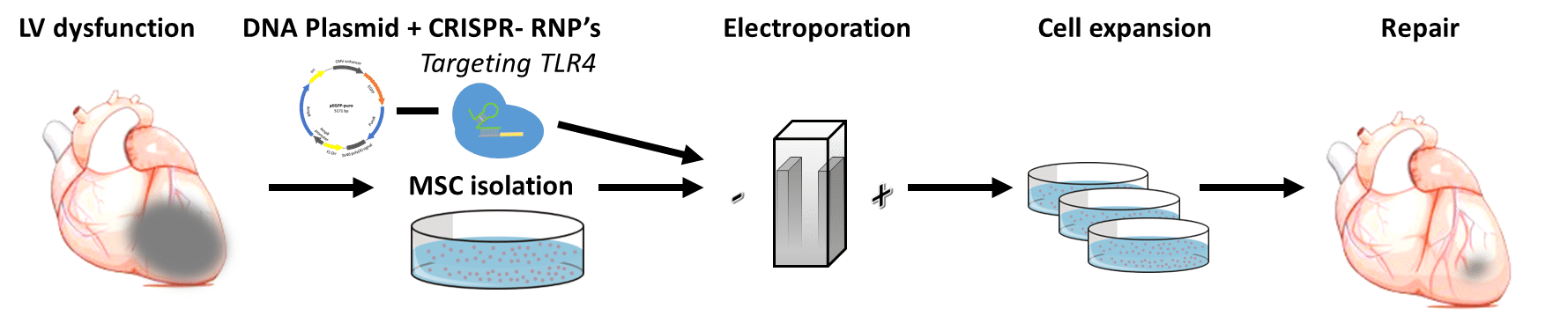
Background and Aim: The environment of the failing and infarcted myocardium drives resident and transplanted mesenchymal stromal cells (MSCs) toward a pro-inflammatory phenotype and restricts their survival and reparative effects in a mechanism mediated by toll-like receptor 4 (TLR4). CRISPR/Cas9 is a promising tool for genome-editing DNA in cells with single-base-pair precision, which raises hopes for therapeutic genome editing in the clinic. We aimed to test the hypothesis that ex-vivo disruption of the human TLR4 gene by CRISPR/Cas9-mediated genome editing would switch MSCs to an anti-inflammatory, reparative phenotype.
Methods and Results: To insert the Cas9 protein into human MSCs without using a viral vector, we used electro-transfection. First, we calibrated the electroporation protocol to work with the cells to define the viable spectrum of electricity tolerated by the cells. For gene editing, we used ribonucleoprotein (RNP), a recombinant Cas9 nucleoprotein attached to a guide-RNA. In our first experiments, we achieved a relatively low (<36%) rate of gene editing, indicated by the amount of addition or deletion (Indels) of nucleotides in the DNA sequence. Next, we further calibrated the electro-transfection protocol with a plasmid that contained both a GFP marker and an antibiotic resistant gene for Puromycin (pEGFP-puro). Co-transfection of the RNPs, together with the pEGFP-puro, provided a negative selection procedure that doubled the rate of edited genes up to 63%. Finally, to determine the effect of TLR4 deletion, we analyzed MSC cytokine secretion, with and without pro-inflammatory, lipopolysaccharide stimulation.
Conclusion: Our preliminary results suggest that human TLR4 gene editing by CRISPR/Cas9 is both feasible and practical. The precise and most efficient genome editing of TLR4 could provide a new strategy for therapeutic application to improve the success of MSC-based cell therapy for infarct repair.

Powered by Eventact EMS