
MOLECULAR IMPRINTING SANS FUNCTIONAL MONOMER
2Pure and Applied Biochemistry, Lund University
Molecular imprinted polymers are commonly prepared by radical copolymerisation of functional monomer(s) and crosslinker in the presence of a template in solution. In the self-assembly approach, the template and functional monomers are first allowed to pre-associate via non-covalent interactions prior to copolymerisation. Among other factors, the imprinting efficiency is influenced by the strength of interaction between the functional groups of the template and the monomers. A functional monomer may not be necessary provided one other component of the polymerisation mixture can be imparted with functional groups capable of interacting with the template. Sibrian-Vasquez and Spivak have shown this to be the case with OMNiMIPs utilising the functional crosslinker N,O-bismethacryloylethanolamine (NOBE).1
This presentation will focus on our efforts and success on molecular imprinting sans functional monomer by utilising functional thermal azo initiator 4,4-Azobis(4-cyanovaleric acid) (ACVA) and functional thiol chain transfer agents (CTAs) thioglycolic acid, thioglycol and cysteamine.
As with other azo radical initiators, ACVA releases N2 gas upon decomposition and 2 equivalents of carboxylic acid-functionalised reactive radicals capable of interacting with the propranolol template and initiating polymerisation of crosslinker TRIM. Results for this study have shown efficient imprinting of (S)-(−)-propranolol and selective rebinding over its enantiomer.
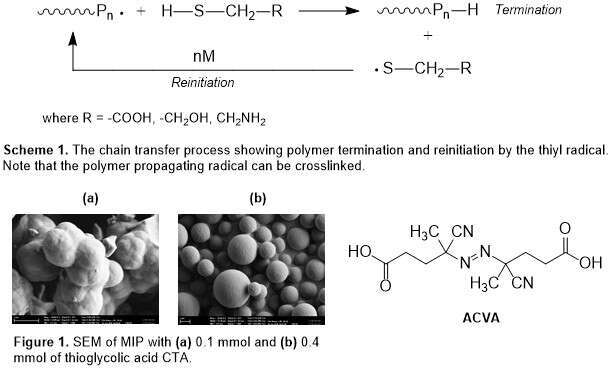
The addition of a functional CTA onto the MIP formulation in lieu of a functional monomer serves two purposes: (1) providing the functional group capable of interaction with the template, and (2) controlling polymer growth and crosslinking which could enhance the imprinting effect. Preliminary binding studies show positive imprinting effect with (R,S)-(±)-propranolol. Further, the bulk system has been observed to behave as a ‘pseudo’ precipitation system, giving discrete microspheres, at higher amounts of CTAs which is most likely correlated to the chain transfer efficiency of these thiols.
Reference
[1] Sibrian-Vasquez, M. and Spivak, D.A. (2004) Molecular Imprinting Made Easy. J. Am. Chem. Soc. 126: 7827-7833.

Powered by Eventact EMS