
INTERFACIAL BEHAVIOUR OF THERMAL RESPONSIVE NANOGELS: STRUCTURAL STUDIES BY NEUTRON REFLECTIVITY
Nanogels are cross-linked networks of polymer chains swollen in a good solvent. In recent years, N-isopropylacrylamide (NIPAM) based thermosensitive nanogels have been widely investigated and shown to have great potential as intelligent materials for biomedical applications, cosmetics, separation techniques to name a few. These materials are surface active, able to reduce the surface 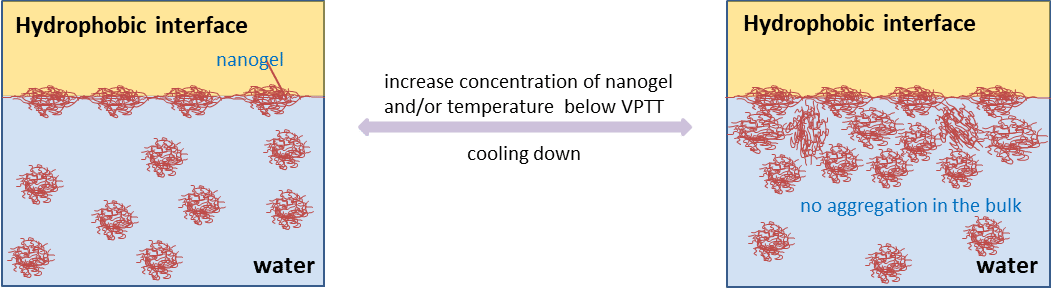 tension of water and giving rise to stable interfaces. Although they are surface active, their behaviour differs substantially from the traditional surfactants, both in the bulk and at interfaces alluding to several possible novel applications. Importantly they form reversible surface aggregates at the expense of bulk aggregation with complex structures, depending on the degree of cross-linkers incorporated. Understanding the adsorption dynamics of those particles and the mechanisms that allow them to stabilize the interfaces are the critical issues that need addressing to achieve tailored particles.
tension of water and giving rise to stable interfaces. Although they are surface active, their behaviour differs substantially from the traditional surfactants, both in the bulk and at interfaces alluding to several possible novel applications. Importantly they form reversible surface aggregates at the expense of bulk aggregation with complex structures, depending on the degree of cross-linkers incorporated. Understanding the adsorption dynamics of those particles and the mechanisms that allow them to stabilize the interfaces are the critical issues that need addressing to achieve tailored particles.
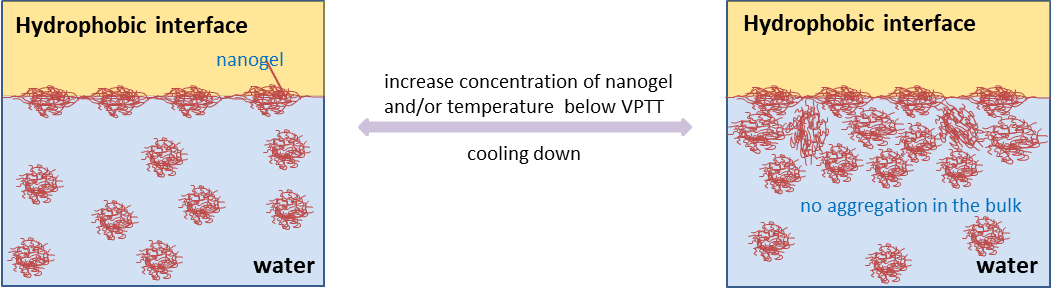
The main objective of this work was to use neutron reflectometry (NR) to probe the interfacial properties of pNIPAM crosslinked nanogels and to examine how the related interfacial behaviour varies as a function of temperature and concentration at the air/water and solid/water interfaces. In the present work a series of pNIPAM nanogels with different amount of cross-linker (N,N`-methylenebisacrylamide, MBA) have been prepared and characterized (size, phase transition and ability to lower water surface tension). NR technique has been used to quantify the nanogels absorbed at the air/water interface and to study their conformational changes as a result of variations in both temperature and concentration. The data obtained contribute to the understanding of how these smart materials stabilize the interface, and to establishing a relationship between their molecular structures and behaviour at interfaces. This new knowledge will contribute to the further applications of these materials.

Powered by Eventact EMS