
SOLID-PHASE SYNTHESIS FOR NANO MIPS COMPATIBLE WITH AQUEOUS ENVIRONMENT: SYNTHETIC ANTIBODY MIMICS FOR CELL TARGETING AND IMAGING
Advanced affinity tools for cell imaging are of particular interest as they can detect, localize and quantify molecular targets. Aberrant glycosylation sites and deregulated expression of certain proteins are promising biomarkers of many human diseases, most notably cancer. However, targeting these biomarkers is often challenging due to a lack of receptor molecules. Herein, we demonstrate biotargeting and bioimaging with fluorescently labeled MIPs on two different cancer biomarkers: hyaluronan and a membrane protein. MIPs were synthesized using a solid-phase synthesis approach in which an epitope of the biomarkers was immobilized on glass beads (as solid support) via click chemistry. This configuration allows an oriented immobilization of the template upon which thermoresponsive MIP nanoparticles were synthesized. The binding sites of the resulting MIPs all have the same orientation, thus MIPs synthesized by the solid-phase approach can be considered analogous to monoclonal antibodies.1,2 Hyaluronan is composed of alternating units of D-glucuronic acid (GlcA) and N-acetyl-D-glucosamine. Thus, azide functionalized-glucuronic acid was immobilized on glass beads bearing alkyne groups. MIP-GlcA (70 nm) as water soluble particles were found to bind selectively extracellular, intracellular and nuclear hyaluronan, as imaged by epifluorescence and confocal microscopies (Figure 1). The specificity of binding was verified with a non-imprinted control polymer and by comparing the staining with a hyaluronan binding protein. For bioimaging the membrane protein, a short peptide (terminal alkyne functionalized) was selected as epitope for immobilization on azide-modified glass beads. The MIP nanoparticles (50 nm) specifically recognized both the template peptide and the whole protein. Cell imaging studies with fluorescent dye-doped MIPs were performed.
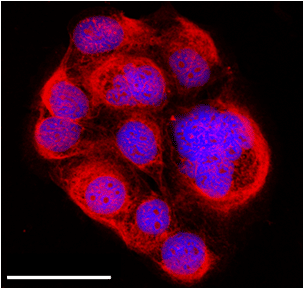
Figure 1 Confocal microscope image showing extracellular, intracellular and nuclear labeling of hyaluronan on fixed human keratinocytes by MIP-GlcA nanoparticles (red). MIP staining with rhodamine (red), nuclear staining with Hoechst (blue), scale bar: 50 µm
[1] Ambrosini S., Beyazit S., Haupt K., Tse Sum Bui B. (2013) Solid-phase synthesis of molecularly imprinted nanoparticles for protein recognition. Chem. Commun. 49: 6746-6748
[2] Xu J., Medina-Rangel P. X., Haupt K., Tse Sum Bui B. (2017) Guide to the preparation of molecularly imprinted polymer nanoparticles for protein recognition, by solid-phase synthesis Methods Enzymol. 590: 115-141

Powered by Eventact EMS