
CAN A MIP BE SELECTIVE TOWARDS THE PRESENCE AND POSITION OF A SINGLE METHYL GROUP? METHYLXANTHINES AS TEMPLATES FOR IMPRINTING.
Caffeine, the main bioactive compound in coffee, is metabolised in the human body in three primary metabolites: paraxanthine, theophylline and theobromine (Figure 1). Quantification of caffeine and its primary metabolites is of high importance due to their distinct biological properties, for applications both in medical research and a variety of chemical industries.1 We have focused on the development of imprinted nanogels that are selective towards these molecules only differing in the positions of methyl groups around the aromatic system.
First, the self-association process of caffeine and its metabolites was investigated by isothermal titration calorimetry (ITC), since the presence of dimers or higher-order clusters would have an effect on the imprinted cavity and therefore, the rebinding properties of the imprinted nanogels. The thermodynamic parameters obtained by ITC were in good agreement with computational calculations performed by our group. All methylxanthines were found to be mainly in a monomeric form at the concentration used for imprinting.2
ITC and NMR spectroscopy were used to evaluate the formation of the pre-polymerisation complex in H2O. A pool of functional monomers was investigated and one was identified that showed a pronounced interaction specifically to paraxanthine. The complex formed between the selected functional monomer and template molecules was evaluated in terms of binding affinity and all thermodynamic parameters were obtained. The nanogel synthesis was optimised in two different solvent systems, H2O and DMSO, using high radical dilution polymerisation.3 Imprinted nanogels were obtained in good yields, ranging from 65 to 100%, with an average particle diameter of 5-10nm. 1H-NMR was employed to quantify monomer incorporation in D2O and DMSO-d6 and compare it with the chemical yields. This allowed to estimate an upper limit for the concentration of binding sites in the polymeric matrices.
The rebinding properties and specificities of the nanogels were determined in water and using High Pressure Liquid Chromatography (HPLC) coupled with UV-Vis spectroscopy. Data on the results obtained thus far will be presented.
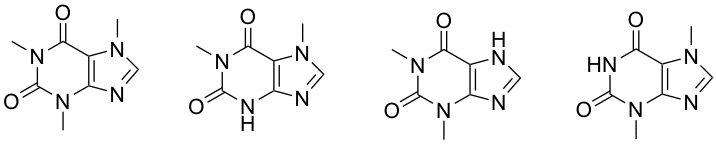
Figure 1 - Caffeine, paraxanthine, theophylline and theobromine (from left to right).
References
[1] Neal L. Benowitz, M.D. (1990) Clinical pharmacology of caffeine. Annual Review of Medicine 41:277-88
[2] R. Bruce Martin (1996) Comparisons of indefinite self-association models. Chemical Reviews, ACS Publications, 96 (8), pp 3043-3064
[3] Andrea Biffis, Neil B. Graham, Georg Siedlaczek, Stefanie stalberg, Günter Wulff (2001) The synthesis, characterisation and molecular recognition properties of imprinted microgels, Macromolecular Chemistry and Physics, 202, 163-171

Powered by Eventact EMS