
The Influence of External Stress/Strain on the Uranium-Hydrogen Reaction
2Ben-Gurion University of the Negev
The extensive exploration of uranium corrosion is driven by the pursuit for the conditions for safe handling and long-term disposal of spent nuclear fuels. It is well documented that during long storage of uranium in sealed containers hazardous hydrogen can be evolved and react exothermally with the uranium to form uranium-hydride powder. It is therefore of importance to understand the factors influencing the uranium-hydrogen reaction, among them the role of applied stress on this reaction.
In the present work we used three-point bending test to examine if different external applied tensile and compression stresses effect differently on the hydride nucleation and growth and differentiate the elastic from the plastic contribution to hydride formation. We found that bending experiments may allow the resolution to answer these questions. Under bending loads, tensile stresses are present on the convex surface whereas compressive stresses prevail at the concave surface. When relieving the bending load, residual elastic stresses are generated on each surface, which are opposite to the stresses developed under the load. The experiments were performed on uranium, but the results may be relevant to other metal-hydrogen reactions.
Fig 1. depicts the pressure change of hydrogen during the reaction with stressed uranium samples. It is therefore evident that increasing the applied tensile stress shortens the incubation periods and promotes the hydride growth centers (GCs) formation. Furthermore, the incubation periods obtained for the samples which were loaded close and above the 0.2% yield stress are shorter by at least a factor of two compared to those loaded below the yield stress. Some moderate increase in the final reaction rate of the loaded samples is also seen, which is related to the accelerated consumption of hydrogen by the GCs.
Microscopic observations of the samples after the reaction are presented in Fig. 2, for the convex (tensile) and the concave (compression) sides of representative specimens (0, 471, 636, and 765 MPa). In comparison, samples under loads which exceed the metal yield stress, exhibit high surface density of GCs formed around the maximal tensile stress on the convex face and only small amount with low surface density on the compressed side. i.e. external tensile stress causes an enhance hydride formation compared to compressive stress.
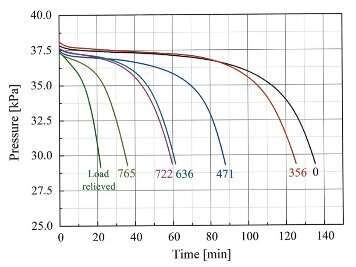
Fig. 1. Hydrogen pressure change vs. time. The maximal tensile stresses (in MPa) at the uppermost edge of the three-point bent specimens are indicated.
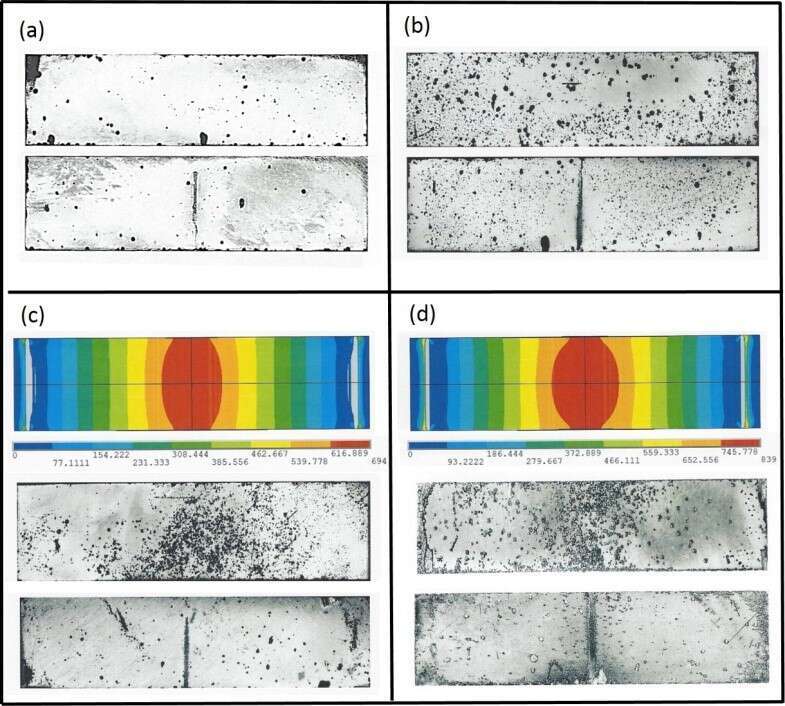
Fig. 2. Optical micrographs of representative samples hydrided under different stress levels. Each face on micrograph includes the tensed side (convex, upper micrograph) and the compressed side (concave, lower micrograph): (a) 0MPa, (b) 471MPa, (c) 636MPa and (d) 765MPa. To illustrate the correlation between the tensile stress and the hydriding location, the calculated stress distribution on the convex side of the samples (636 and 765MPa) are presented.

Powered by Eventact EMS