In recent years, major breakthroughs in understanding and controlling the adaptive immune system have led to a new emergent personalized medicine field, the immunotherapy. Generally, a person immune system is exploited in the treatment against a disease, mostly many kinds of cancer, either by amplifying or by supressing an immune response.
Due to their central role in mounting an immune response, T-cells are promising candidates for immunotherapy treatments. T cells probe the surface of antigen presenting cells (APCs) for cognate foreign antigens in order to initiate an adequate immune response. This unique system requires the detection of a very weak signal (very rare foreign peptide - antigen) in the presence of considerable noise (abundant self peptides). In spite of its importance, much remains to be learned about the physical mechanisms that lead to T cell activation. Current models of mechanisms of T cell activation include dynamic molecular aggregation, conformational changes (mechanical effects) and kinetic segregation of inactivating molecules from sites of activation. Experimental evidence exist for each mechanism and thus imply that all mechanisms are involved simultaneously in the complex process of T cell activation.
The goals of this research work is to (1) quantify dynamic molecular patterning during cell activation, focusing on the role of protein tyrosine phosphatases in T cell activation, and (2) to resolve and quantify the effects of mechanical and
topographical properties over synapse creation and T cell activation.
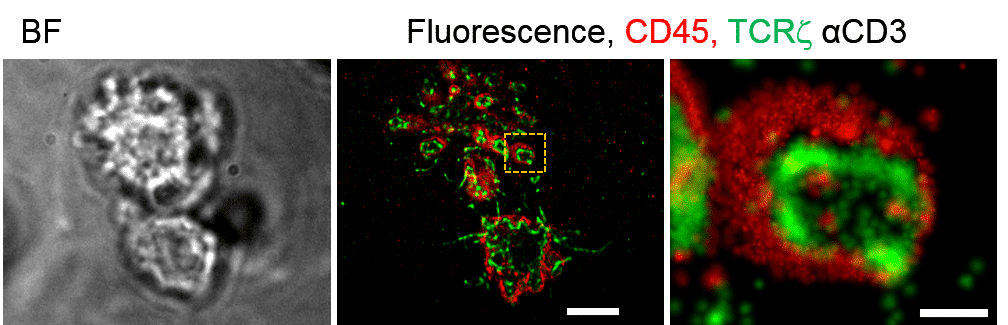
For that purpose, we used live-cell multi-color single-molecule-localization microscopy (SMLM) to identify and study the dynamic separation between T cell antigen receptor (TCR) and CD45 glycoprotein phosphatases in early forming cell contacts under TCR-activating and non-activating conditions. Atomic-Force Microscopy (AFM) and SMLM identified these
contacts with engaged microvilli and enabled to characterize their morphology, rigidity, and dynamics. We found the dynamic separation is with a complex nature as the TCR is both activated and de-activated by the CD45. Thus, although seperated by activation, the CD45 remains in the area of the signaling complex.
Furthermore, we utilize AFM measurements on living cells to study the relation, if any, between morphology and rigidity, in particular in T cells. We found there is a strong correlation between the two as the topography peaks, i.e, the microvili pretrusions, are with higher values of rigidity. We hypothesized that it might be the driven force in tight adhesion contacts at the immune synapse (IS).
Moreover, we developed a physical model and simulations that accurately captured the dynamic TCR-CD45 separation and the rigidity-topography relation under the experimental conditions.
Altogether, we expect that our findings and understanding the nature of the physical interactions that take place at the IS will shed light on the critical process of T cell activation. By so we pave the way for new novelty immunotherapy
treatments.
References:
Yair Razvag, Yair Neve-Oz, Julia Sajman, Meital Reches and Eilon Sherman
Nanoscale kinetic segregation of the T cell antigen receptor and CD45 in engaged microvilli
facilitates early T cell activation. Nature Communications 2018 9:732
Yair Razvag, Yair Neve-Oz, Eilon Sherman and Meital Reches
Morphology rigidity correlation regulates T cell activation
Submitted for publication

