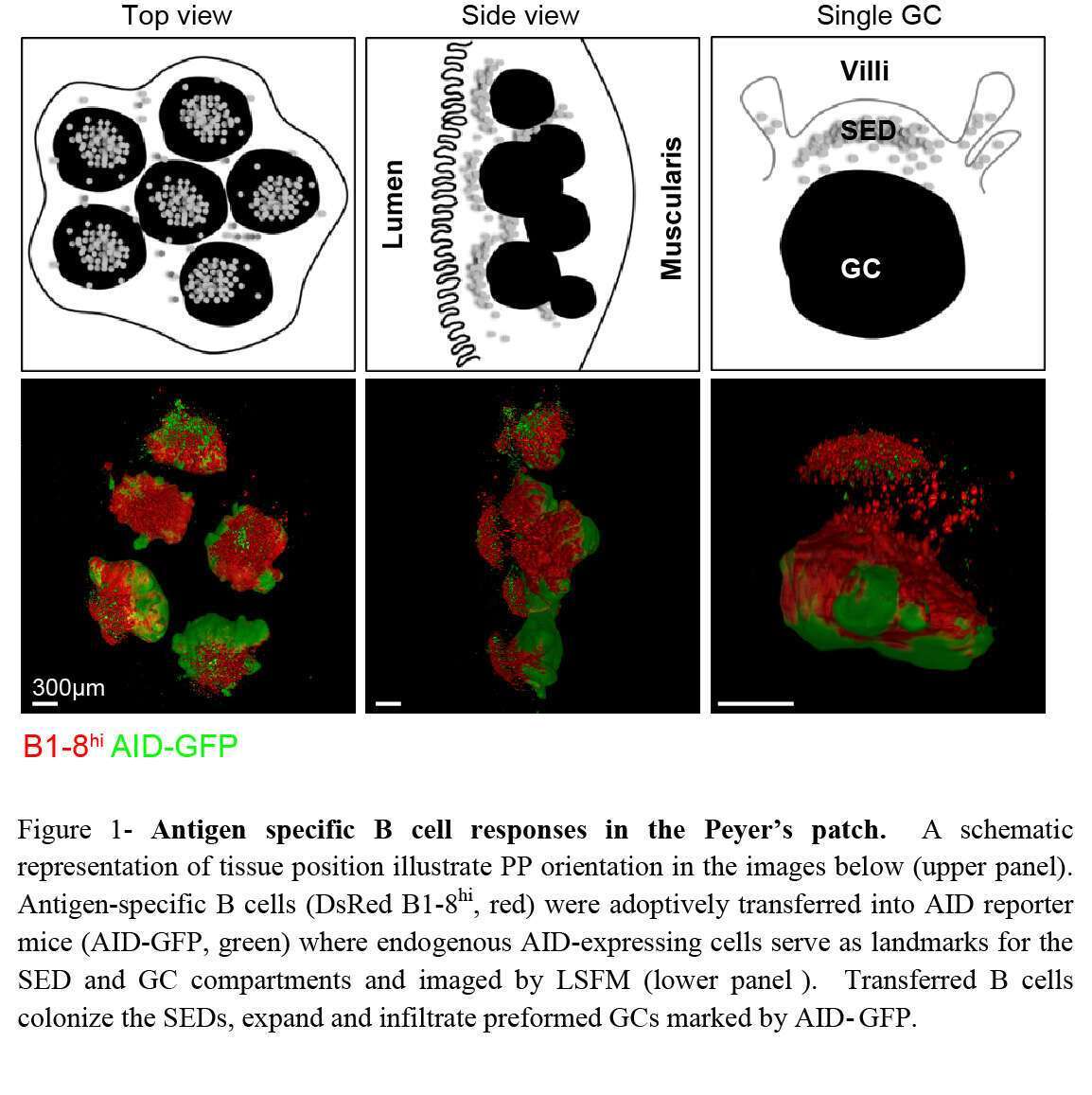
The intestinal immune system is constitutively exposed to dietary and microbiome-derived antigens. Maintenance of host defense requires a balance between immune effector responses and tolerance under homeostatic conditions which is mainly achieved through production of antibodies in intestinal tissues (1). Effective antibody immune responses and establishment of long-lasting protection from invading pathogens depend on an increase in antibody affinity over time. This process, known as antibody affinity maturation, takes place in germinal centers (GC) where B cells undergo diversification by somatic hypermutation (SHM) and affinity-based clonal selection (2, 3). Mucosal-derived antigens are delivered into microanatomical structures within the PP known as subepithelial domes (SED) where B cells and antigen presenting cells reside (4). Following antigen uptake in the SED, antigen specific B cells migrate into the GC for further clonal expansion and diversification (5). Since the SED has a dome-like shape, it is unfeasible to capture its full structure neither by conventional immunohistochemistry nor by two photon laser scanning microscopy (TPLSM) methods. We combined whole organ imaging by light sheet fluorescence microscopy (LSFM) with an oral immunization model that allows us to examine the mechanism of B cell clonal selection in PPs. We find that B cells are subjected to stringent clonal selection by T cells during SED departure, resulting in increased clonal dominance in GCs revealed by single cell immunoglobulin sequencing. Opposed to GCs, SED B cell clones are highly diversified, indicating that clonal selection takes place during SED departure. Our results demonstrate that the threshold for GC entry following oral immunization in PPs is different from the responses elicited by conventional vaccines in draining lymph nodes. These findings have wide implications for design of oral vaccination strategies.
References
- Reboldi A, Cyster JG (2016) Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol Rev 271(1):230–245.
- Victora GD, Nussenzweig MC (2012) Germinal Centers. Annu Rev Immunol 30(1):429–457.
- EISEN HN, SISKIND GW (1964) VARIATIONS IN AFFINITIES OF ANTIBODIES DURING THE IMMUNE RESPONSE. Biochemistry 3:996–1008.
- Reboldi A, et al. (2016) IgA production requires B cell interaction with subepithelial dendritic cells in Peyer’s patches. Science (80- ) 352(6287):aaf4822.
- Bergqvist P, et al. (2012) Re-utilization of germinal centers in multiple Peyer’s patches results in highly synchronized, oligoclonal, and affinity-matured gut IgA responses. Mucosal Immunol 6(1):122–135.