A central property of developing plant and animal tissues is their ability to form a diversity of folded patterns and to adopt curved shapes through mechanical instabilities that break the planar symmetry of growing epithelia[1,2–4]. The folding of growing epithelia can result from external mechanical constraints that restrict in-plane expansion[5]. Morphological changes can also occur in unconstrained sheets[6], where the emergence of wrinkles is associated with excess growth of the margins relative to the center of the sheet. This mechanism explains the shape of leaves and flowers[6–8]. In contrast to the shaping of growing tissues, the deformation of animal embryos during gastrulation often proceeds largely in absence of tissue growth and relies on active contractility[9–11]. In most cases, the mechanisms which are responsible for tissue folding are usually studied in cell epithelia (layer(s) of cells). In that case, shape deformations have been observed, when a contractile epithelium was subjected to anisotropic contraction [13] as well as in the case when it was coupled to an elastic substrate. [10] This presents a major difficulty for studying the intrinsic effect of actomyosin contractility on tissue folding in vivo where the external deformations and substrate cannot be controlled.
In recent work, we show that suspended, contractile actomyosin gel sheets can provide a good model system for studying contraction-induced folding. [12] We show that contractile acto-myosin gel sheets behave as a poroelastic material, where a flow of fluid is generated during contraction. We also observed that the acto-myosin sheet can spontaneously buckle and that the buckling instability resulted from system self-organization and the spontaneous emergence of density gradients driven by the active contractility. These new findings demonstrate that buckling can be spontaneously generated by myosin activity and does not require mechanical coupling to the environment or pre-imposed gradients in the material properties of the sheet. The latter effect has been shown to be responsible for buckling in synthetic systems prepared with inhomogeneous crosslinkers densities or elastic moduli [14].
We fabricated symmetric, circular thin suspended contractile elastic acto-myosin sheets of controllable extent and elastic properties [12] by polymerizing actin in the presence of the strong passive cross-linker fascin and clusters of myosin II motors that act as active crosslinkers. Contractile elastic gels formed only if actin polymerization occurred in the presence of the motors and passive cross-linkers. [15–17] The polymerization process was performed in chambers with a high aspect ratio. Specifically, the sheets had initial radii (lateral extension) of about 1/2 and an initial thickness of typically a hundred (vertical extension). This corresponds to an aspect ratio, . The actomyosin gels spontaneously contracted through the activity of myosin motors with no need for external stimuli, save for the presence of ATP in solution. Contraction occurred only above a myosin concentration threshold [16]. For lower concentrations, the mechanical stresses generated by the motors were apparently too weak to overcome the gel’s elasticity, whereas for higher motor concentrations the gel ruptured. [16,17]
The anisotropy in the gel extensions decoupled the contraction processes in the vertical and lateral directions. Namely, contraction started in the vertical direction and only when vertical contraction was essentially terminated, did the contraction begin along the lateral direction, thereby generating 2d actomyosin gel sheets of finite thickness. We found that during lateral contraction, the sheet thickness remained essentially constant. The actomyosin gels thus correspond to materials with an effective Poisson ratio ; as discussed below, this is due to the flow of water out of the material as it contracts which is the signature of poroelasticity [10,12] of combined fluid flow and gel deformation as opposed to gel elasticity alone. At advanced stages of planar contraction, the sheet spontaneously started to buckle. The buckling instability resulted from system self-organization and was due to the spontaneous emergence of density gradients (highest at the gel edge and lowest in the center) driven by the active contractility that persisted in steady state. Buckling occured when contraction of the gel periphery could not follow the contraction in the gel bulk. This is reminiscent of surfaces under the constraint of a fixed perimeter that is longer than the perimeter of a circle of the same surface area. The emerging folds were directed perpendicular to the boundary and had a roughly sinusoidal shape (Figure 1). The characteristic wavelength of the folded pattern was proportional to the sheet thickness, e.g., for a sheet thickness of with a corresponding wavelength of . Note that the macroscopic buckling phenomenon shown here occurs on a much larger length scale compared to the mesh size of the gel ( at the end of the contraction process) and does not result from the buckling of individual filaments observed in dilute actomyosin networks in the presence of weak cross-linkers such as α-actinin. [18-20)
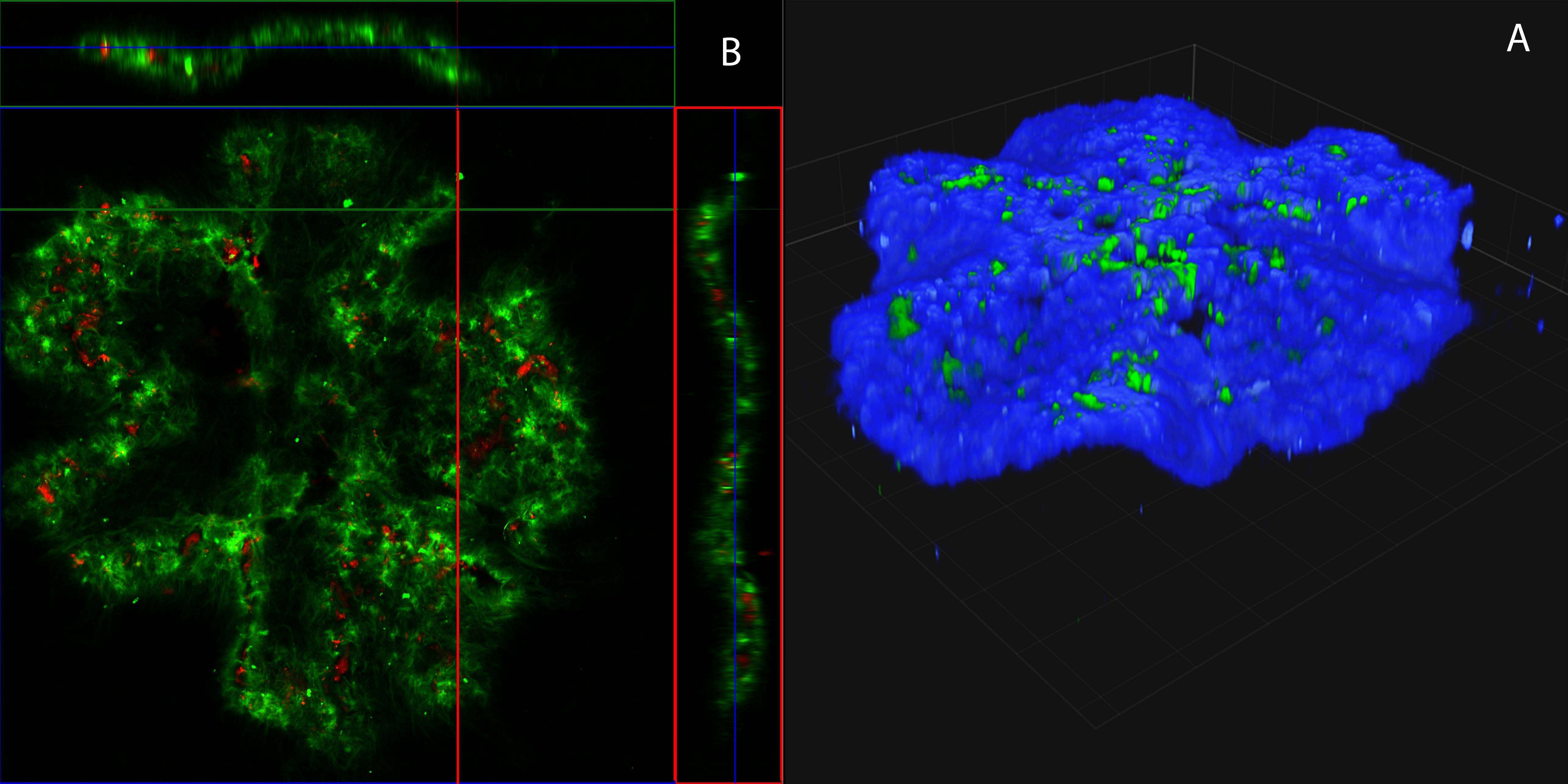
Figure 1. Buckling of an acto-myosin sheet . A) Laser scanning confocal micrograph of a three-dimensional view of a buckling gel at steady state(actin-blue,myosid green). B) Laser scanning confocal fluorescence micrographs of the actin gel in (A) in the xy-plane (top view), the xz-plane (top side view), and yz-plane (left side view)(actin-green,myosin-red). Side views (xz and yz-planes) are measured along the white lines. Scale bars: (horizontal) and (vertical) (insets of A) and (B).

Figure 2: Actomyosin network contraction on XY plain. epifluorescence microscope. time scale 5 min. scale bar – 200um.
References
[1] C. P. Heisenberg, Y. Bellaïche, Cell 2013, DOI 10.1016/j.cell.2013.05.008.
[2] B. Li, Y.-P. Cao, X.-Q. Feng, H. Gao, Soft Matter 2012, 8, 5728.
[3] J. A. Gemmer, S. C. Venkataramani, Nonlinearity 2012, 25, 3553.
[4] B. Audoly, A. Boudaoud, Phys. Rev. Lett. 2003, 91, 86105.
[5] A. E. Shyer, T. Tallinen, N. L. Nerurkar, Z. Wei, E. S. Gil, D. L. Kaplan, C. J. Tabin, L. Mahadevan, Science 2013, 342, 212.
[6] J. Dervaux, M. Ben Amar, Phys. Rev. Lett. 2008, 101, 68101.
[7] U. Nath, B. C. W. Crawford, R. Carpenter, E. Coen, Science (80-. ). 2003, 299, 1404.
[8] H. Liang, L. Mahadevan, Proc. Natl. Acad. Sci. U. S. A. 2011, 108, 5516.
[9] C. P. Heisenberg, Y. Bellaïche, Cell 2013, DOI 10.1016/j.cell.2013.05.008.
[10] E. Hannezo, J. Prost, J.-F. Joanny, Proc. Natl. Acad. Sci. 2014, DOI 10.1073/pnas.1312076111.
[11] B. He, K. Doubrovinski, O. Polyakov, E. Wieschaus, Nature 2014, DOI 10.1038/nature13070.
[12] Y. Ideses, V. Erukhimovitch, R. Brand, D. Jourdain, J. Salmeron Hernandez, U. R. Gabinet., S. A. Safran, K. Kruse, A. Bernheim-Groswasser, Submitted 2017.
[13] A. Livshits, L. Shani-Zerbib, Y. Maroudas-Sacks, E. Braun, K. Keren, Cell Rep. 2017, DOI 10.1016/j.celrep.2017.01.036.
[14] Y. Klein, E. Efrati, E. Sharon, Science 2007, 315, 1116.
[15] F. Backouche, L. Haviv, D. Groswasser, A. Bernheim-Groswasser, Phys. Biol. 2006, DOI 10.1088/1478-3975/3/4/004.
[16] Y. Ideses, A. Sonn-Segev, Y. Roichman, A. Bernheim-Groswasser, Soft Matter 2013, DOI 10.1039/c3sm50309g.
[17] J. Alvarado, M. Sheinman, A. Sharma, F. C. MacKintosh, G. H. Koenderink, Nat. Phys. 2013, DOI 10.1038/nphys2715.
[18] M. P. Murrell, M. L. Gardel, Proc. Natl. Acad. Sci. 2012, DOI 10.1073/pnas.1214753109.
[19] M. Lenz, T. Thoresen, M. L. Gardel, A. R. Dinner, Phys. Rev. Lett. 2012, DOI 10.1103/PhysRevLett.108.238107.
[20] X. Xu, S. A. Safran, Phys. Rev. E - Stat. Nonlinear, Soft Matter Phys. 2015, 92, 1.

