Alarming signs of the malaria parasite resistance to current drug treatments highlight the need for identification of efficient targets to improve present antimalarial therapies. Residing in a human red blood cell the malaria parasite consumes hemoglobin and digests it in an organelle called the digestive vacuole. Hemoglobin digestion liberates large quantities of heme. This iron-containing molecule is toxic to the parasite. The parasite detoxifies heme by turning it into inert hemozoin crystals. This waste-disposal mechanism is a promising drug target. How heme is crystallized remains uncertain, but current models predict very different rates of crystallization. To this end we have developed a unique correlative X-ray absorption cryo-tomography and X-ray fluorescence cryo-microscopy method that enables us to determine the distribution and concentration of chemical elements relative to the three-dimensional cellular structure of instantly frozen snapshots of the malaria parasites in red blood cells.1 Our measurements of heme crystallization rate and our discovery of considerable amounts of hemoglobin in the digestive vacuole suggest an assembly line process of heme detoxification: heme monomers liberated from hemoglobin are dimerized via the heme detoxification protein (HDP) and the dimers crystallize into hemozoin.2 According to this model, the rates of heme monomer release, heme dimerization and hemozoin formation must closely match. Thus, two targets for drug development become apparent: inhibition of HDP, a subject of ongoing studies, and inhibition of hemozoin crystallization. Both targets will lead to accumulation of toxic heme monomers and self-poisoning of the parasite. Indeed, quinoline-family drugs are believed to act by targeting hemozoin crystallization. Specifically, molecular simulation studies predict that the drugs adsorb onto the surface of hemozoin crystals thereby inhibiting their growth3,4. In order to investigate this model, we used our X-ray cryo-microscopy method to quantitatively map the distribution of bromoquine, an analog of the classical chloroquine drug, within the malaria parasites. To avoid altering distribution of chemical elements due to sample preparation, the parasites were rapidly vitrified by cryo-freezing avoiding chemical fixation, staining, embedding or sectioning. Our results strongly support the predicted affinity of bromoquine to hemozoin crystals (see Figure 1). Interestingly, we also detect accumulation of bromoquine at the nucleus of the parasite indicating an additional antimalarial activity.
In conclusion, our correlative X-ray cryo-microscopy method establishes a new approach for the measurement of element-specific concentrations within intact cellular ultrastructure at high spatial resolutions of ~50 nm or better. This, in-turn, enables us to further investigate heme metabolism, antimalarial drug action and resistance in the malaria parasites.
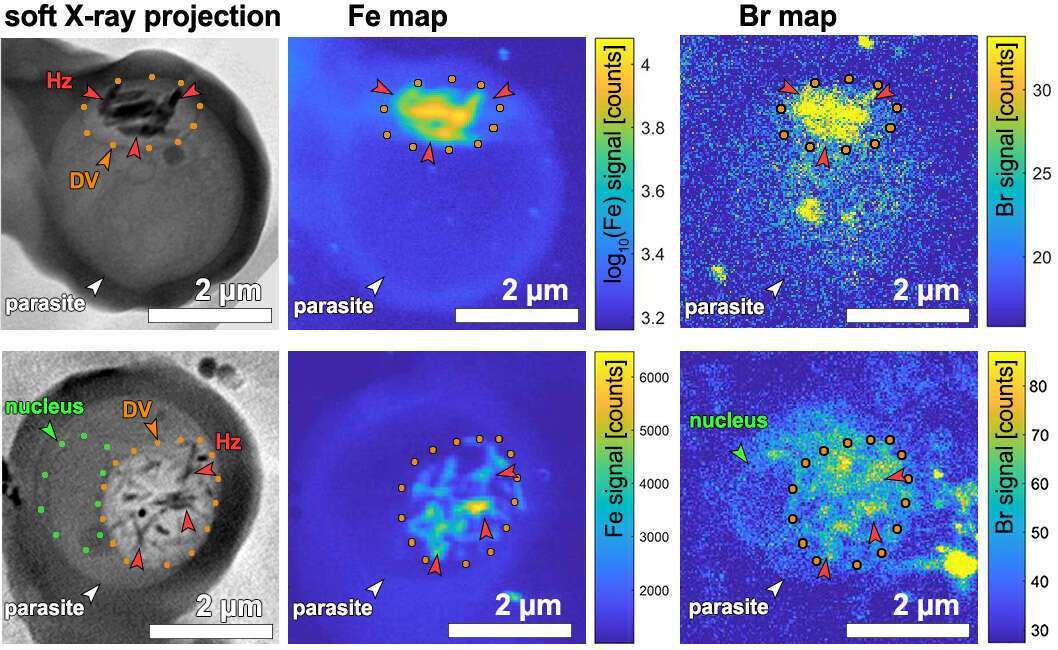
Figure 1. Soft X-ray cryo-tomography (SXT) re-projection images and X-ray fluorescence maps of two malaria parasite-infected red blood cells. Left: SXT maps. Center: iron (Fe) maps. Right: bromine (Br) maps. DV – digestive vacuole delineated with orange dots containing iron-dense hemozoin (Hz) crystals.
References:
1 Kapishnikov, S. et al. Scientific Reports 7, 802 (2017).
2 Kapishnikov, S. et al. Scientific Reports 7, 7610 (2017).
3 Dubar, F. et al. ACS Chemical Biology 6, 275-287 (2011).
4 Buller, R. et al. Cryst Growth Des 2, 553-562 (2002).

