Plant pathogenic bacteria employ the type III secretion system (T3SS) to deliver sets of protein effectors into the cytoplasm of the host cell. These effectors promote bacterial virulence through alteration of the host cell metabolism and/or suppression of host defense responses (Mansfield 2009). Acidovorax citrulli is a Gram-negative bacterium that causes bacterial fruit blotch of cucurbits, a devastating disease that threatens watermelon and melon production worldwide (Burdman and Walcott 2012). Based on genetic, biochemical and host preferential association, the A. citrulli population is divided into three groups: group I includes strains that are moderately to highly aggressive on a wide range of cucurbits strains (isolated mainly from melon and other non-watermelon cucurbits), group II strains are highly aggressive on watermelon (mainly isolated from watermelon), and group III strains possess relatively weak virulence on melon, watermelon and pumpkin (Walcott et al. 2000, Eckshtain-Levi et al. 2014). Sequence analysis of genes encoding type III-secreted effectors (T3Es) from a global population of strains revealed that the A. citrulli groups significantly differ each from the other, based on effectors’ repertoire and sequences (Eckshtain-Levi et al. 2014). Gene aopW1, homologous to Pseudomonas syringae hopW1-1, is an example of a T3E gene that may be involved in the specialization of A. citrulli groups to different hosts. aopW1 (1455 bp) is relatively conserved among the three A. citrulli groups, with the exception of a 138-bp hypervariable region (HVR) located at nucleotide positions 439 to 576. Heterologous expression of aopW1 genes in yeast revealed that the group I and III versions exerted strong growth inhibition and lethality, while the group II version of this effector had only a subtle effect. In this sense, similar effects were shown when this effector was expressed in planta. Besides, confocal microscopy analysis revealed that AopW co-localizes with chloroplasts, actin cytoskeleton, endoplasmic reticulum (ER) and plant endosomes. However, the group I AopW1, but not the group II AopW1, disrupts actin cytoskeleton in vivo.
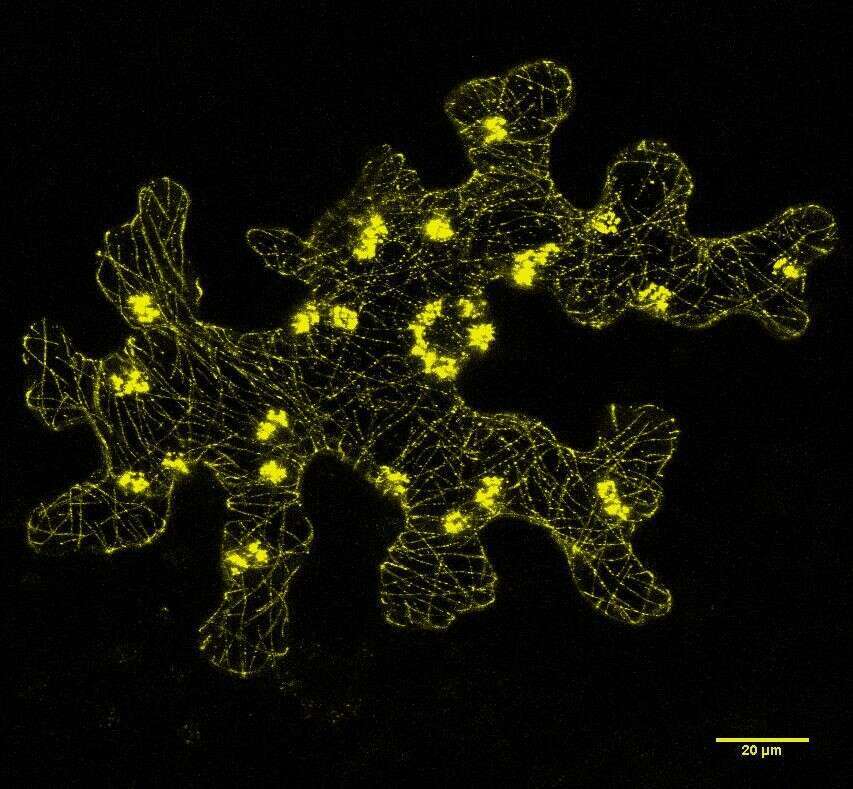 |
Figure 1: Subcellular localization of the AopW1 group I effector in Nicotiana benthamiana leaves.
References: Mansfield JW. (2009) Mol Plant Pathol 10:721-34. Burdman S, Walcott R. (2012) Mol Plant Pathol 13:805-815. Walcott RR, Langston DB, Sanders FH, et al. (2000) Phytopathology 90:191-196. Eckshtain-Levi N, Munitz T, Zivanovic M, et al. (2014) Phytopathology 104:1152-62.
This work has been supported by grant US-4734-14C from the US-Israel Binational Agricultural Research and Development (BARD) Fund.


