
STED Nanoscopy Assisted by Small Metal Nanoparticles – New Advances
We show ~100nm resolution using low intensity in a stimulated-emission-depletion (STED) microscope using 20nm gold spheres coated with fluorophores in aqueous environment, along with up to 3-fold reduction of bleaching rate.
The limit that diffraction puts on imaging was considered as one of the most fundamental problems in wave physics. This limit was broken in the early 2000’s in the context of fluorescence microscopy, eventually resulting in the awarding of the Nobel Prize of 2014. The most prominent super-resolution technique is probably stimulated-emission-depletion (STED) nanoscopy [1], which offers both superb resolution as well as fast acquisition times. To achieve the highest resolution, STED nanoscopes use high laser powers, often requiring expensive laser sources and also risking increasingly strong photobleaching. Recently [2], we showed that metal nanoparticles can be used to improve the performance of STED nanoscopes with a potential resolution improvement by more than an order of magnitude, or equivalently, depletion intensity reductions by more than 2 orders of magnitude; these come along with a strong photostabilization (i.e. reduction of photobleaching) due to the shortened fluorophore lifetime, which reduces the absolute number of bleaching events.
The first proof-of-concept of this approach [3], referred to as nanoparticle assisted STED (NP-STED) nanoscopy, was performed with 150nm gold shells, and demonstrated only moderate super-resolution levels. It was also performed in an oil environment, rather than in water, as appropriate for biological samples.
In this contribution, we report on a range of additional yet unpublished results. We use 20nm gold spheres coated with fluorescent silica in aqueous environment, and a much shorter STED wavelength, of 595nm. First, we demonstrate deep super-resolution with the hybrid nanoparticles, down to ~100nm (see Fig. 1(left)), at up to 2 times lower intensities compared with a standard STED. No boiling of the aqueous environment is observed within this range, indicating the method’s applicability to imaging of biological media.
Second, we demonstrate up to a 3-fold reduction of the bleaching rate in both confocal and STED modes (see Fig. 1(right)), thus, providing the first confirmation of the second part of the NP-STED theory.
Further miniaturization of the NP-STED label and optimization of fluorophore location can enable far better performance. This approach is especially suitable for parallel STED systems where the lack of sufficient power is a limiting factor in enabling the scan speed increase. Our hybrid nanoparticles could also enable additional imaging capabilities, such as photothermal imaging, SPASing, nonlinearity-based super-resolution imaging [4], and most importantly, correlated light-electron imaging, which is currently highly desired within the microscopy community. Our particles can also enable combination of STED with the multitude of procedures that rely on insertion of few nm plasmonic nanoparticles to cells, creating a versatile bio-tool. These include photothermal therapy, membrane perforation, gene therapy treatments, stimulation, monitoring and signaling in neurons, tweezing, drug delivery, as well as Biodiagnostics based on spherical nucleic acids [5], which are quite similar to the NPs used in this study, all of which could benefit greatly from the substantial improvement of the imaging resolution offered by NP-STED.
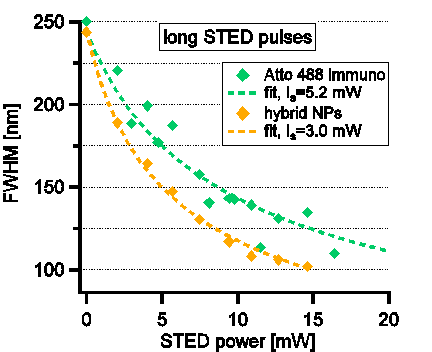
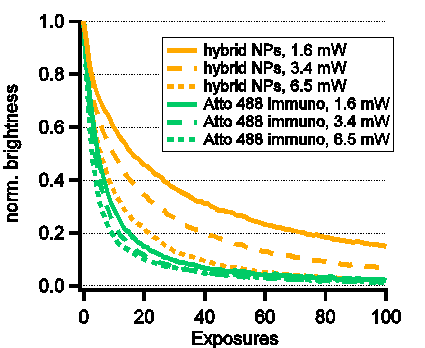
Fig. 1 (left) resolution as a function of incoming STED intensity. Intensity required for maximal resolution of ~100nm is more than twice lower with the hybrid nanoparticles. (right) brightness reduction curves due to photobleaching. A clear slowing of bleaching is observed when the hybrid nanoparticles are used.
References
[1] T.A. Klar, S. Jakobs, M. Dyba, A. Egner and S.W. Hell. Proc. Nat. Acad. Sci. U.S.A 97, 8206-8210 (2001)
[2] Y. Sivan et al., ACS Nano 6, 5291-5296 (2012); Y. Sivan, Appl. Phys. Lett. 101, 021111 (2012)
[3] Y. Sonnefraud, H.G. Sinclair, Y. Sivan, M.R. Foreman, C. Dunsby, M.A.A. Neil, P. French, S.A. Maier. Nano Lett. 14, 4449 (2014)
[4] S.-W. Chu et al., ACS Photonics 1, 32-37 (2013); S.-W. Chu et al., Phys. Rev. Lett. 112, 017402 (2014).
[5] C. Mirkin, Nature 382, 607-609 (1996); N.L. Rosi and C. Mirkin, Chem. Rev. 105, 1547-1562 (2005).
Powered by Eventact EMS