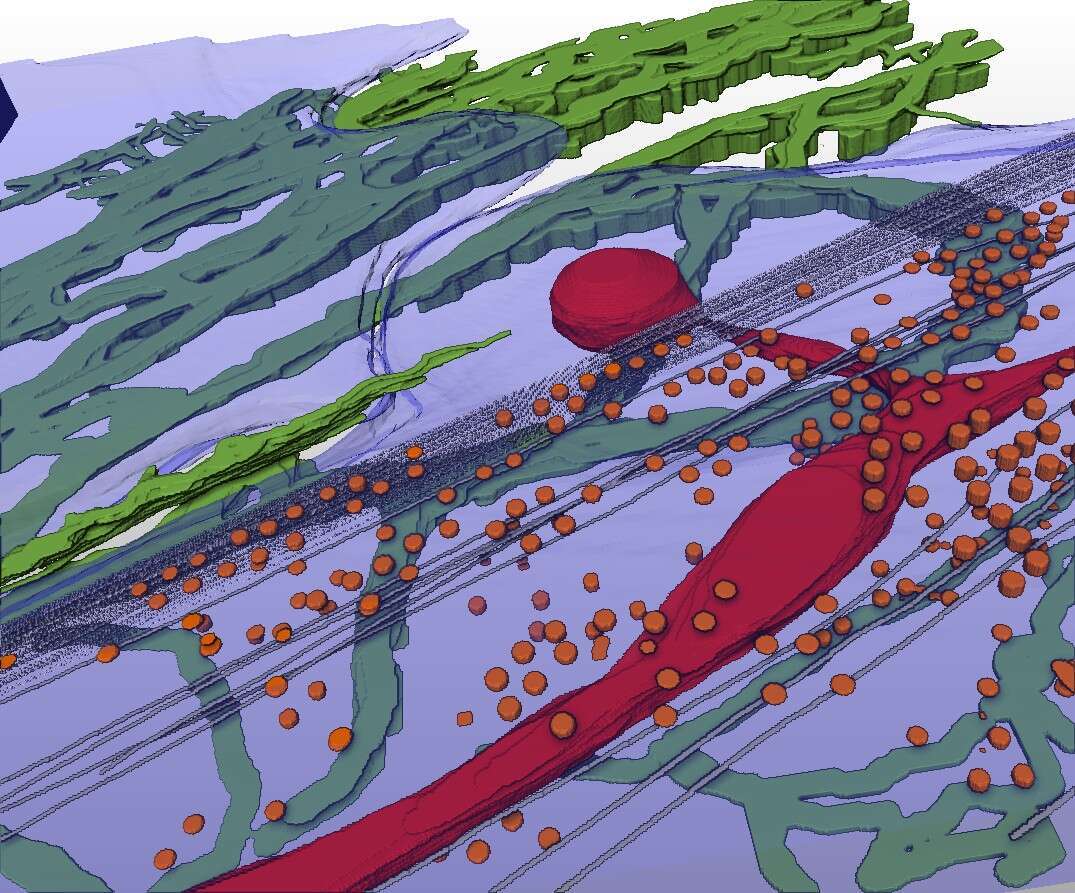
Cryo-scanning transmission electron tomography (CSTET) is a novel method for creating three dimensional images of biological samples at nanometer resolution, while keeping the samples in their native hydrated state, without fixation, dehydration, or other perturbation[1–3]. We apply this technique to map the organization of the extracellular matrix (ECM) in cell cultures while simultaneously visualizing the underlying cell ultrastructure. Such snapshots can lead to insights about how the cells deposit and interact with the ECM, processes crucial for healthy physiology and which are perturbed by diseases such as cancer and myopathy[4,5]. The most abundant ECM components deposited by fibroblasts, collagen VI and fibronectin, were labeled with gold beads for contrast and identification. This enabled us to see collagen VI networks and fibronectin filaments together with intracellular organelles, cytoskeletal elements, and cell surface features such as caveolae (figure 1). Microfibril network patterns of collagen VI were analyzed quantitatively, showing the characteristic repeats of globular domains within individual fibrils and presenting novel information on fibril organization and turnover in the cell microenvironment. Specifically, cells appeared to migrate into collagen VI that had been widely dispersed in the cell vicinity following secretion, and collagen VI network fragments containing a few dozen globular repeats were taken up and recycled into large intracellular vesicles. This work demonstrates that CSTET is complementary to super-resolution light microscopy for the study of extracellular matrix in cell biology, highlighting labeled extracellular elements against a backdrop of unlabeled but morphologically identifiable cellular features with nanometer resolution detail.
References:
[1] S.G. Wolf, L. Houben, M. Elbaum, Cryo-scanning transmission electron tomography of vitrified cells, Nat Meth. 11 (2014) 423–428.
[2] S.G. Wolf, P. Rez, M. Elbaum, Phosphorus detection in vitrified bacteria by cryo-STEM annular dark-field analysis, J. Microsc. 260 (2015) 227–233.
[3] S.G. Wolf, Y. Mutsafi, T. Dadosh, T. Ilani, Z. Lansky, B. Horowitz, S. Rubin, M. Elbaum, D. Fass, 3D visualization of mitochondrial solid-phase calcium stores in whole cells, Elife. 6 (2017) 1–18.
[4] L.K. Zamurs, M.A. Idoate, E. Hanssen, A. Gomez-Ibanez, P. Pastor, S.R. Lamande, Aberrant mitochondria in a bethlem myopathy patient with a homozygous amino acid substitution that destabilizes the Collagen VI α2(VI) chain, J. Biol. Chem. 290 (2015) 4272–4281.
[5] W.K. You, P. Bonaldo, W.B. Stallcup, Collagen VI ablation retards brain tumor progression due to deficits in assembly of the vascular basal lamina, Am. J. Pathol. 180 (2012) 1145–1158.

