Some animals use mirrors rather than lenses to form images1. Such mirrored eyes are extremely efficient at collecting light and are found in marine animals living in dim-light habitats. A fascinating example of such an eye is found in decapod crustaceans: the reflective superposition compound eye (Fig. 1A). Previously it was known that each eye contains two types of reflector: (i) an image-forming “distal mirror” in the upper part of the eye which focuses light onto the retina (comprised of the photosensitive rhabdoms); (ii) an intensity-enhancing “tapetum reflector” underlying the retina which back-scatters light onto the rhabdoms, giving the retina a second chance to capture dispersed light. We recently reported that, in freshwater crayfish and prawns, these reflectors are formed from highly reflective crystals of isoxanthopterin2 – a previously unknown optically functional bio-crystal.
Here, we report the discovery and characterization of a third “proximal” reflector in the eye of the whiteleg shrimp L. vannamei (Fig. 1B-F). The reflector is located below the tapetum within the nerve layer of the eye (lamina ganglionaris). The reflector is separated from the retina by a thick pigment layer and does not contribute to image-formation, since light impinging along the eye-axis will be absorbed before reaching the reflector. Instead, we suggest the reflector acts to camouflage the eye by back-scattering off-axis light away from the highly conspicuous eye-pigments. Using X-ray micro-CT, light and fluorescent microscopy and cryo-SEM in combination with confocal Raman microspectrometry, we show that this reflector is constructed from crystalline nanoparticles of isoxanthopterin and functions by diffuse light-scattering. We also report the identification of isoxanthopterin crystals in numerous infraorders across the decapoda including Astacidea, Caridea and Dendrobranchiata, confirming that this material is found widely in decapod crustaceans. These results call for the investigation of crystalline pteridines more widely in the animal kingdom.
In conclusion, we unearth a remarkable and unique multi-functional natural optical device containing three types of reflector associated with three optical functions: image-formation, light-enhancement and camouflage. Understanding how organisms control the formation and arrangement of organic crystalline materials to manipulate light could pave the way for the development of novel bio-inspired organic optical materials.
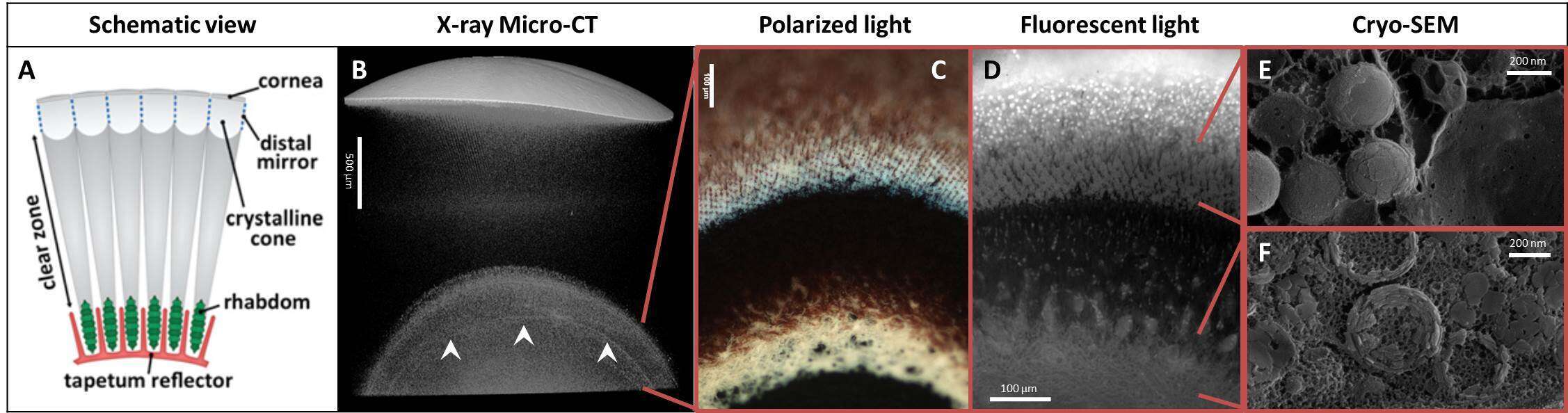
Figure 1: Millimeter-to-nanometer scale architecture of the tapetum and proximal reflectors. (A) Schematic of the reflecting compound eye viewed along the optic axis. (B) X-ray microCT scan of part of an eye, showing four high contrast features: the cornea (uppermost), distal mirror (immediately below cornea), tapetum (lower, between red lines) and proximal reflector (arrowheads, between red lines). (C) Polarized light micrograph of the highly birefringent tapetum (upper) and proximal (lower) reflectors. (D) DAPI stained fluorescent microscopy image of the tapetum and proximal reflectors, showing cell nuclei (tapetum layer), isoxanthopterin auto-fluorescence (tapetum and proximal layers) and myelin auto-fluorescence (proximal layer). (E) - (F) Cryo-SEM images from the tapetum (E) and the proximal reflector (F) regions displaying crystalline isoxanthopterin nanoparticles.
References:
1 Palmer BA et al., Science. 2017 358, 1172-1175.
2 Palmer BA et al., Proc. Natl. Acad. Sci. U. S. A. 2018 115, 2299-2304.

