
THE BUTANOLOGENTIC CELLULOSOME OF CLOSTRIDIUM SACCHAROPERBUTYLACETONICUM
2Department of Life Sciences, Ben-Gurion University of the Negev, Beer-Sheva, Israel
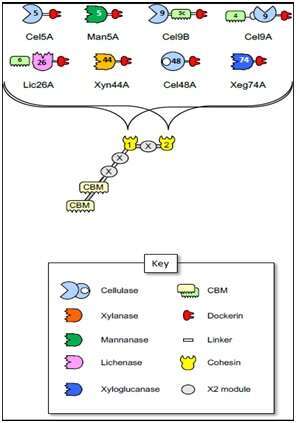
Clostridium saccharoperbutylacetonicum (“Berry”) is a mesophilic, anaerobic butanol-producing bacterium originally isolated from soil 1. It was recently reported that C. saccharoperbutylacetonicum possesses multiple cellulosomal elements 2. Theoretically, this cellulosome is among the smallest known in nature. It thus has a “minimalistic” cellulosome with only 8 dockerin-bearing enzymes and 2 cohesins (Cohs) of the scaffoldin in the sequenced genome 2. In this study, each cellulosome-related module (cohesin, dockerin, enzyme and carbohydrate-binding modules) was cloned, expressed and purified, and the recombinant components were tested for enzymatic activity on cellulosic substrates and binding activity using ELISA-based techniques. All the enzymes were tested for their comparative enzymatic activity on 7 different substrates, and the data revealed 4 cellulases, a xylanase, a mannanase, a xyloglucanase, and a lichenase. All dockerin-containing enzymes interacted with the Coh-2 module, while Coh-1 was more restricted in its interaction pattern. We also tested the ability of C. saccharoperbutylacetonicum to produce bio-butanol from 4 different agrowaste sources (potato peel, apple pomance, brewer’s spent grain, and coffee silverskin). Our preliminary results showed that Berry produced the highest amount of butanol when grown on tryptone-yeast extract-acetate media with potato peel as the carbohydrate source, yielding a total product of 5.4 g/L butanol.
- H. Motoyoshi, Process for producing butanol by fermentation. In Google Patents: 1960.
- B. Dassa, I. Borovok, V. Lombard, B. Henrissat, R. Lamed, E. A. Bayer, S. Moraïs, Microorganisms 2017, 5, 74.
Powered by Eventact EMS