
Functionalized Titanium Dioxide Nanoparticles for Targeting and Disrupting Amyloid fibrils
Amyloidoses are a family of diseases characterized by abnormal protein folding that leads to aggregation. They accumulate to form fibrillar plaques which are implicated in the pathogenesis of Alzheimer, prion, diabetes type II and other diseases. Despite extensive research efforts devoted to plaque aggregates inhibition there is yet no cure for this phenomenon.
In recent years there has been growing interest in utilizing titanium and its alloys in biomedical applications. Various surface modification that produce porous, adhesive, bioactive coatings have been developed. Titanium oxides (titania) are also being developed for photothermal and photodynamic treatments.
Inspired by this, we set to explore the effect of functionalized titania nanoparticles in combination with external UV stimuli, as potential photothermal ablating agents against amyloids. Titania nanoparticles were coated with bi-functional dihydroxy-phenylalanine (DPA) derivatives to gain targeting properties. In conjunction with UV-radiation these nanoparticles may selectively destroy the vicinity of their target.
Titania modified 5 nm nanoparticles coated with DPA were further conjugated to the amyloid-targeting Congo Red (CR). These Titania-DPA-CR nanoparticles were found to target mature amyloid fibril of both amyloid- (A 1-42 a.a) and prion peptide (PrP fragment, 106-126 a.a). Moreover, irradiation of the peptides in presence of the modified nanoparticles decreased the aggregate content and oligomer fraction. This work provides new insights into the use of modified titania nanoparticles for amyloid plaque targeting and photothermal destruction. It may shed light on future modifications and functionalization of titania nanoparticles for different applications.
References
1. Gitelman, A. & Rapaport, H. Bifunctional Designed Peptides Induce Mineralization and Binding to TiO2. Langmuir 30, 47164724 (2014). 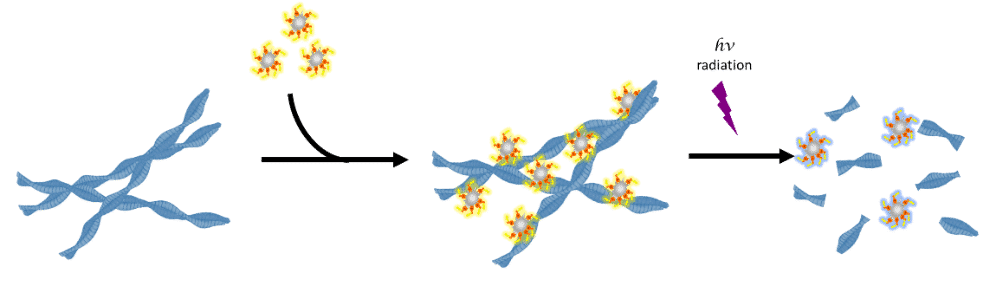
Powered by Eventact EMS