In 2015, we changed the protocol for early detection and treatment of early onset sepsis (EOS) based on the 2010 recommendations of the Centers for Disease Control and Prevention (CDC).
In this study, we prospectively evaluated the new protocol.
Inclusion criteria
Gestational age of 35 weeks or more
Newborns receiving antibiotic treatment during the first 72 hours after delivery
Newborns whose mothers were treated with intra partum antibiotic therapy (IAT)
We prospectively followed for one year (May 2015 – April 2016) all newborns that met the inclusion criteria.
Results
A total of 7062 newborns were born . Out of them, 1345 (19%) newborns met study criteria.
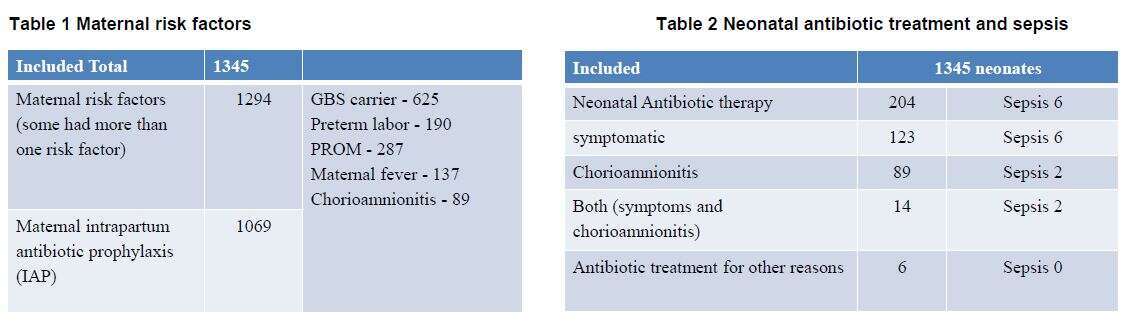
Of the included neonates, 89/1345 (6.62%) had maternal suspected chorioamnionitis, two (2.25%) had proven EOS, but were also symptomatic. The RR for symptomatic newborn to have EOS was 10.9 (CI 3.5-33.39, P<0.0001) and the NNT was 22.6, while the RR for newborn with suspected maternal chorioamnionitis was 5.03 (CI 1.03-24.6 P=0.045) and the NNT was 55.5. Symptomatic newborns were at 2.2 increased risks to have EOS compared with newborns with suspected maternal chorioamnionitis.
Conclusions
The current protocol (CDC2010) was found to be effective and safe in diagnosing and treating all cases of EOS. All newborns with proven sepsis were symptomatic. According to the protocol, we also treated 75 asymptomatic newborns whose mothers had suspected choroiamnionis.
We suggest that asymptomatic newborns born to mothers with suspected chorioamnionitis should not be automatically treated with antibiotics. However, more studies are needed to confirm the above data.

