
Invited
A twisted tale: Helically-locked tethered acenes
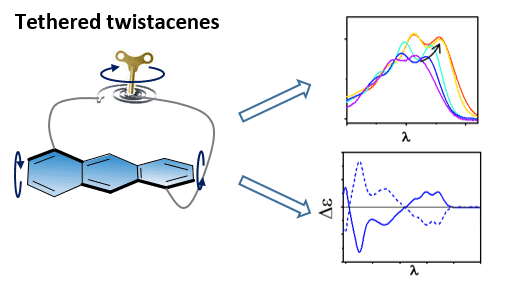
Acenes, which can be viewed as one-dimensional graphene nanoribbons, are an important class of organic electronic materials. While many linear acenes suffer from low stability and solubility, twisting acenes out of planarity improves both their stability and solubility. In addition, such twisting is expected to alter their electronic and optical properties, and to induce chirality. However, it is difficult to isolate the effect of twisting from the substituent effect. Moreover, many twistacenes (twisted acenes) readily racemize in solution.
Recently, we introduced a new series of twistacenes having an anthracene backbone diagonally tethered by an n-alkyl bridge, which induces a twist of various angles. This allows us to systematically monitor the effect of twisting on electronic, optical and chiroptical properties. We find that absorption is bathochromically shifted with increasing twist, while fluorescence quantum efficiency drops dramatically. The tethered twistacenes were isolated in their enantiomerically pure form, displaying strong chiroptical properties and anisotropy factor (g-value), which are enhanced with increasing twist. No racemization was observed even upon prolonged heating, rendering these tethered twistacenes suitable as enantiopure helical building units for π-conjugated backbones.
Reference:
A. Bedi, L. J. W. Shimon, and O. Gidron, J. Am. Chem. Soc. 2018, 140, 8086-8090.
Powered by Eventact EMS