
Flash
Fluorescent probes incorporating the chromophore of green fluorescent protein for the detection of HER-2 breast cancer marker
2Department of Pharmacy, Pharmaceutical and Medicinal Chemistry,, Saarland University, Saarbrücken, Saarbrücken, Germany
3Faculty of Life Sciences & Institute of Nanotechnology, Bar-Ilan University, Ramat-Gan, Israel
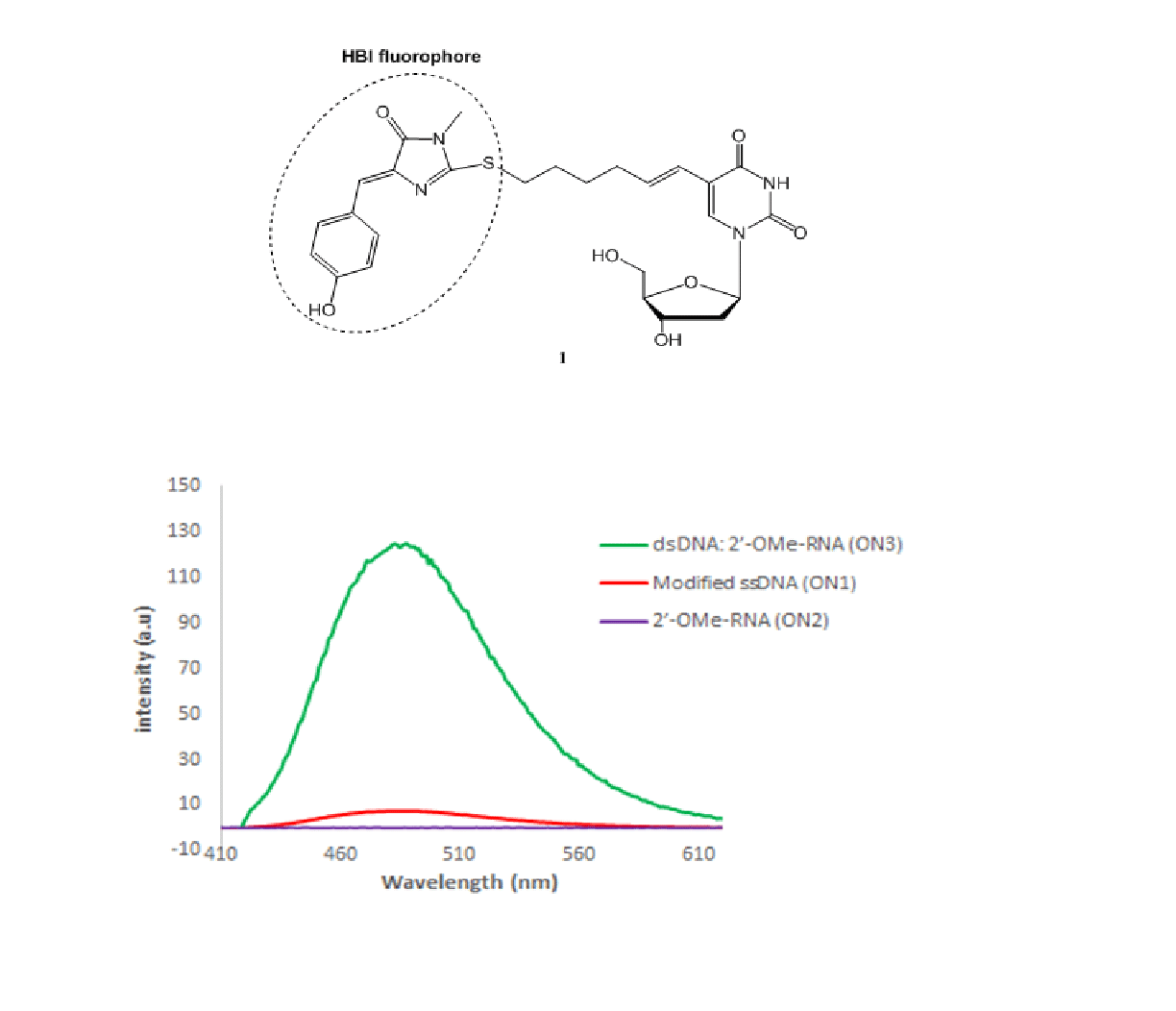
Diagnosis and treatment of breast cancer can be greatly enhanced and personalized based on the quantitative detection of mRNA markers. Here, we targeted a fluorescent probe to detect specifically the HER-2 mRNA breast cancer marker. We have selected the chromophore of the Green Fluorescent Protein (GFP), 4-hydroxybenzylidene imidazolinone (HBI), as a fluorophore covalently bound to an oligonucleotide probe and potentially capable of intercalating with a probe-RNA duplex. Since the HBI chromophore fluoresces only upon planar fixation of the benzylidene and imidazolinone rings, and in solution radiationless internal conversion quenches fluorescence, we expected to obtain a large signal/background ratio of the probe upon hybridization with target nucleic acid. For this purpose, we have synthesized and characterized the photophysical properties of Nucleoside-Intercalator Conjugate (NIC) monomer 1 to be incorporated into an oligonucleotide. Indeed, in the highly viscous glycerol used to mimic the reduced conformational flexibility of the intercalated HBI chromophore, 1 displayed quantum yield of 0.31 and brightness of 20600 M-1cm-1, while no fluorescent signal was observed in methanol. As a proof of concept, we have synthesized a 20-mer oligonucleotide probe incorporating 1 at position 15, targeting nucleotides 721–741 of HER-2 breast cancer mRNA marker. A 16-fold enhancement of HBI emission intensity upon hybridization with the complementary RNA vs that of the oligonucleotide probe alone indicated the presence of target oligonucleotide and proved the intercalation of the chromophore (quantum yield 0.52; brightness 23500 M-1cm-1).
Powered by Eventact EMS