
Invited
Metal-binding as a new approach for peptoids folding
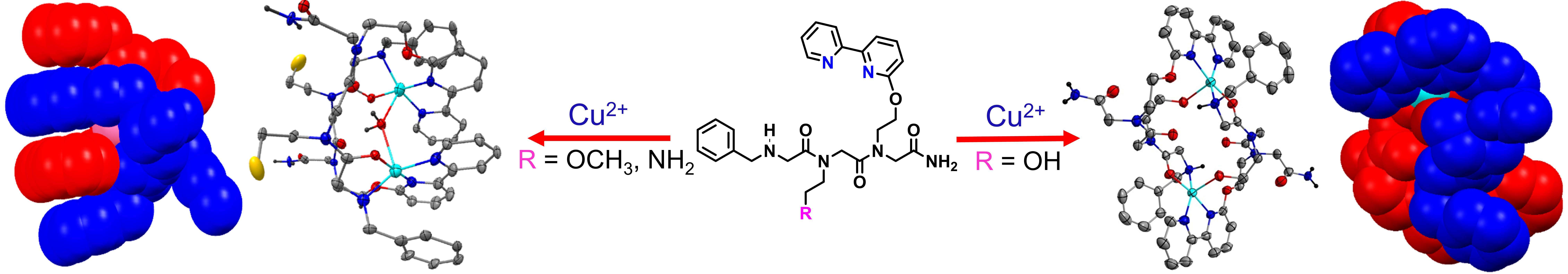
Peptoids, N-substituted glycine oligomers, are an important class of peptide mimics that are generated from primary amines rather than from amino acids. Their facile and efficient synthesis on solid phase support enables the incorporation of various functional groups at specified N-positions along their spine including metal-binding ligands and catalysts.1 Peptoids can adopt polyproline type helices if the majority of their sequence consists of chiral bulky pendent groups. Such side-chains are structure inducers but they have no functional value. Herein I will present the inclusion of several metal-binding groups in one peptoid oligomer as a new platform to develop functional helical peptoids and supramolecular peptoid architectures. Thus, I will describe the coordination of two metal ions to unstructured peptoids incorporating several chelators as side chains. For the first time, we demonstrate by spectroscopic techniques, DFT calculations and X-ray analysis peptoids folding upon metal binding to form helical structures.2 Interestingly, binding of Cu2+ to one coordination site initiates a conformational change that in turn facilitates the coordination of Zn2+ (or Co2+) ion to a second site, demonstrating a unique example of positive allosteric cooperativity in peptide mimics.2b Moreover, X-ray diffraction analysis revealed that metal-binding in short peptoids lead to exceptional, highly symmetric, cyclic structures by the self-assembly of two peptoid molecules with two Cu(II) ions, including the first examples of aqua-bridged dinuclear double-stranded peptoid helicates.3
References:
- (a) T. Zabrodski, M. Baskin, P. Jeya Kaniraj, G. Maayan Synlett 2015, A1. (b) P. Jeya Kaniraj, G. Maayan, Chem. Commun. 2015, 11096. (c) M. Baskin, G. Maayan, Chem. Sci. 2016, 7, 2809. (d) T. Ghosh, P. Ghosh, G. Maayan, ACS Catal. 2018, 8, 10631.
- (a) L. Zborovsky, A. Smolyakova, M. Baskin and G. Maayan, Chem. Eur. J., 2018, 24, 1159. (b) M. Baskin, H. Zhu, Z.-W. Qu, J. Chill, S. Grimme, G. Maayan, Chem. Sci. 2019, DOI: 10.1039/C8SC03616K.
- T. Ghosh, N. Fridman, M. Kosa, G. Maayan, Angew. Chem. Int. Ed. 2018, 57, 7703.
Powered by Eventact EMS