
A concise synthetic route to dual C- and P-chiral hydroxyalkyl phosphine oxides and phosphines
Department of Organic Chemistry, Israel Institute for Biological Research, Ness-Ziona, Israel
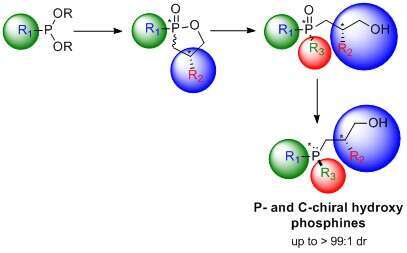
A facile, stereoselective method for the synthesis of both carbon- and P-chiral phosphine oxides and phosphines bearing a hydroxyl chelating arm was developed. A carefully designed oxaphospholane was constructed via tandem Arbuzov- intramolecular cyclization reaction, using commercially available compounds. Regioselective ring opening alkylation/arylation provided optically active phosphine oxides within two synthetic steps. An additional step of stereospecific deoxygenation produced P-chiral tertiary phosphines in high dr.
Powered by Eventact EMS