
Helically-locked tethered twistacenes
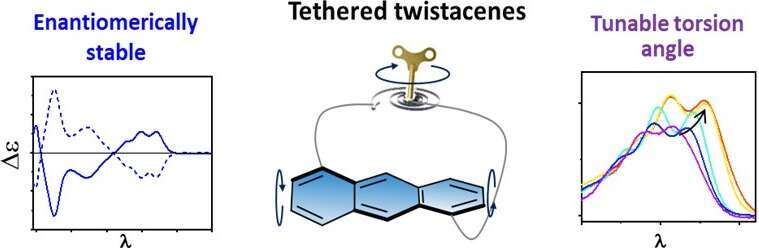
Twisting linear acenes out of planarity affects their electronic and optical properties, and induces axial chirality. However, it is difficult to isolate the effect of twisting from the substituent effect. Moreover, many twistacenes (twisted acenes) readily racemize in solution.1 Backbone locking can be achieved by molecular tethering, such as was previously applied to achieve bending of larger polyaromatic hydrocarbons.2 Here, we introduce a series of twistacenes having an anthracene backbone diagonally tethered by an n-alkyl bridge, which induces a twist of various angles.3 This allows us to systematically monitor the effect of twisting on electronic and optical properties. We find that absorption is bathochromically shifted with increasing twist, while fluorescence quantum efficiency drops dramatically. The tethered twistacenes were isolated to their enantiomerically pure form, displaying strong chiroptical properties and anisotropy factor (g-value). No racemization was observed even upon prolonged heating, rendering these tethered twistacenes suitable as axially chiral building units for π-conjugated backbones.
References
1. J. Lu, D. M. Ho, N. J. Vogelaar, C. M. Kraml, R. A. Pascal, J. Am. Chem. Soc. 2004, 126, 11168.
2. P. R. Nandaluru, P. Dongare, C. M. Kraml, R. A. Pascal, L. N. Dawe, D. W. Thompson, G. J. Bodwell, Chem. Commun. 2012, 48, 7747.
3. A. Bedi, L. J. W. Shimon, O. Gidron, J. Am. Chem. Soc. 2018, 140, 8086.
Powered by Eventact EMS