
Invited
Metal-free catalytic reductive cleavage of enol ethers
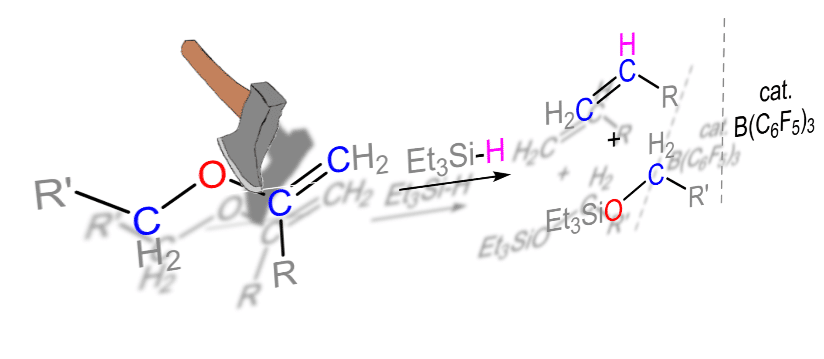
In contrast to the well-known reductive cleavage of the Alkyl−O bond, the activation of Alkenyl−O is more challenging and way more rare, especially by metal-free approaches. Unexpectedly, a Alkenyl−O bond was cleaved when enol ethers were reacted with Et3SiH in the presence of a catalytic amount of B(C6F5)3, leading to alkoxysilanes and alkenes. Supposedly, this reaction is a result of a B(C6F5)3 catalyzed tandem hydrosilylation reaction of the C=C double bond of the alkenyl group, followed by Lewis acid catalyzed, silicon-assisted β-elimination. This hydrosilylation-elimination reaction sequence gives, formally, a nucleophilic vinylic substitution (SNV) of alkoxy group by the hydride of the Et3SiH. This reactivity was probed with different alkyl and silyl enol ethers and the mechanism of this cleavage reaction was studied both experimentally and by density functional theory (DFT) calculations.
Powered by Eventact EMS