
Crossover-tolerant coated platinum catalysts in hydrogen/bromine redox flow battery
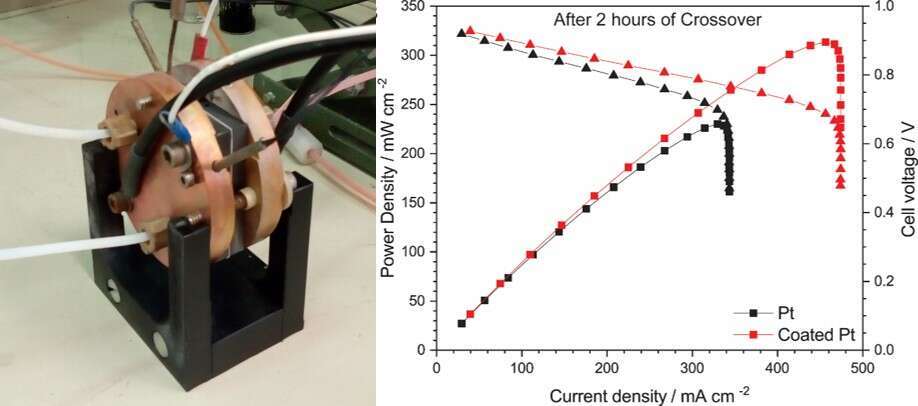
The hydrogen-bromine redox flow battery`s (H2-Br2 RFB) advantages of high energy capacity, high round-trip conversion efficiency and low cost, make it an optimal candidate for large-scale energy storage systems. The crossover of bromide species through the membrane degrades the performance of the H2-Br2 RFB by poisoning the catalyst responsible for the hydrogen evolution and oxidation reactions. Herein we propose the new concept of a selective catalyst coating layer that mitigates the effect of bromide crossover. The polymerization of dopamine on the catalyst surface yields a nanoscale conformal polydopamine layer which acts as a semi-permeable barrier to block bromide species. The H2-Br2 RFB with the coated catalyst shows a low capacity fading of 6% at 300 mA cm-2 after exposure to 4.5 M charged electrolyte for 2 hours. Even the beginning of life polarization curves show the benefit of catalyst coating with a high peak power of ~550 mW cm-2. Hence, the catalyst coating opens a way to solve the crossover issue in H2-Br2 RFB technology.
Powered by Eventact EMS