
Contributed
Selective benzylic H/D exchange using unsolvated hydroxide
2Wolfson Department of Chemical Engineering, Technion – Israel Institute of Technology, Haifa, Israel
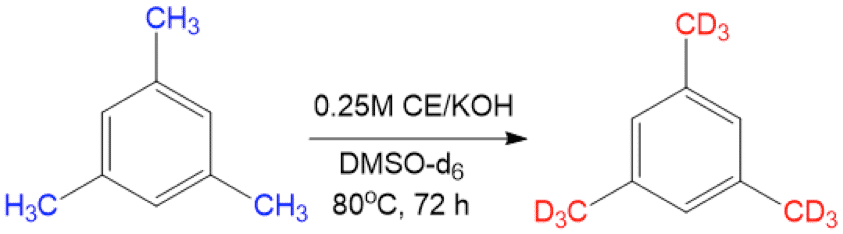
Selective replacement of hydrogen atoms with deuterium is an important chemical transformation mainly used for mechanistic and kinetic studies in organic chemistry. In addition, the use of deuterium has been shown to provide increased metabolic lifetime in drug molecules while maintaining similar pharmacologic profile. Typically, the synthesis of deuterated compounds involves the early use of simple deuterated reactants or expensive metal-catalyzed regioselective H/D exchanges. During the course of our studies on the effect of water solvation on the nucleophilicity and basicity of hydroxide, we noticed the disappearance of benzylic hydrogens from the 1H-NMR in molecules with high pKas such as mesitylene. Further research demonstrated that this simple, yet extremely reactive base is capable of inducing regioselective benzylic H/D exchange in a few hours using DMSO-d6 as deuterium source, reaching very high conversions. In this talk, I’ll present the scope of the reaction, tolerated functional groups, and mechanistic studies, as well as an example of late stage H/D exchange in a complex drug molecule.
Powered by Eventact EMS